Research
Since 2007, the Laboratory of Ophthalmology (UA) has collaborated with the Centre for Cell Therapy and Regenerative Medicine and the Department of Ophthalmology, UZA on the project “Translational research in ophthalmology - regenerating the anterior cornea though standardized limbal stem cell transplantation: a phase I/II clinical trial”. This research funded by the IWT TBM has completed its phase I/II trial with a 68% success rate and a Belgian multicenter study funded in January 2014. Through transplantation of corneal epithelial stem cells cultivated ex vivo on standardized amniotic membranes, a treatment option is provided for patients suffering from visual loss due to corneal epithelial efficiency caused by: burns, multiple surgeries, genetic diseases such as aniridia, etc. While successful in cases where only the superficial (epithelial) layer needs replacing, there are many conditions where stromal scarring or endothelial dysfunction requires replacement of part of, or the entire, cornea. Current research in the Department of Ophthalmology, Visual Optics and Visual Rehabilitation is focused on the development of new treatment options and the optimisation of existing techniques.
Recent topics
Tissue engineering in ophthalmology: regenerating the anterior cornea using self-aligning human recombinant collagen nanoscaffolds and corneal epithelial stem cells.
Development of a biomimetic cornea combining additive manufacturing and stem cell technologies.
Optimisation of cell culturing techniques with a focus on corneal endothelial cells.
Tissue engineering in ophthalmology: regenerating the anterior cornea using self-aligning human recombinant collagen nanoscaffolds and corneal epithelial stem cells.
Background
The human cornea consists of three cellular layers: the superficial epithelium, the avascular and transparent stroma and the endothelium which consists of a single cell layer. The limbus is the anatomical junction between the cornea and the conjunctiva, and it holds the Limbal Epithelial Stem Cells (LESC). The key role of the LESC is to regenerate the corneal epithelium and maintain the physiological barrier between the vascular conjunctiva and the avascular cornea. Trauma to the limbus and LESC results in a deficiency of the limbal stem cells, which subsequently leads to conjunctival overgrowth of the cornea and corneal scarring. This results in corneal neovascularisation and opacification with reduced vision, pain and photophobia in the affected eye.
Recent research has proven the feasibility and the advantages of a minimal surgical procedure, where a small limbal biopt is prelevated from living donor. This biopsy is then placed in culture on a biological membrane, and the most frequently used is the Human Amniotic Membrane (HAM) (figure 1). However, since the amniotic membrane is a biological membrane with strong inter-individual characteristics, the culturing process of the LESC is difficult to standardise and it holds potential health risks for the acceptor. Further research is needed to develop an alternative membrane with superior characteristics.
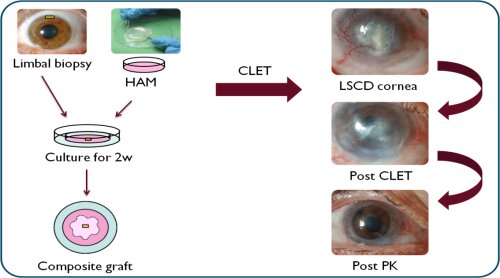
Figure 1: In Cultivated Limbal Epithelial Transplantation (CLET) a small limbal biopsy is being cultivated on top of a carrier material, after which the composite graft is being transplanted to the patient’s diseased eye. The Human Amniotic Membrane (HAM) is the natural membrane most commonly used as the carrier material.
Outline of the project
The goal of this research is to develop an adequate and adjusted membrane (starting from Self-Aligning Human Recombinant Collagen type I) to replace the use of HAM in corneal tissue engineering. The recent introduction of the Self-Aligning Human Recombinant Collagen makes the development of a synthetic, transparent and mechanically stable membrane possible, onto which LESC can be cultured to regenerate the corneal stroma after an initial trauma. In comparison to HAM, we expect to develop a membrane with an improved transparency, higher mechanical stability, better bio-integration and a reduced microbiological susceptibility. The effect of collagen cross linking and surface modifications will contribute in the development of the most optimal membrane. The refractive index, light scattering, toxicity, mutagenicity, permeability, in vitro phenotyping of cultured cells and cell-membrane-interactions will be investigated. The membrane will be validated in vivo. All obtained data will be compared to the HAM, which is the current gold standard.
First experiments have indicated that collagen membranes have a (i) refractive index closer to that of the human cornea, (ii) are more transparent (figure 2), and (iii) provide 20 times less straylight compared to that of the current golden standard (HAM). In vitro experiments have furthermore shown that LESC can successfully be cultivated on collagen membranes (figure 3), and that cultivated primary cells keep expressing their phenotypical LESC-markers (figure 4).

Figure 2: A collagen membrane compressed between 2 glass slides. It is to expected that the transparency, optimal refractive index and reduced straylight will increase patient’s post-operative visual outcome.
Figure 3: LESC outgrown form a limbal biopsy A collagen membrane compressed between 2 glass slides. It is to be expected that the transparency, optimal refractive index and reduced straylight will increase patient’s post-operative visual outcome.
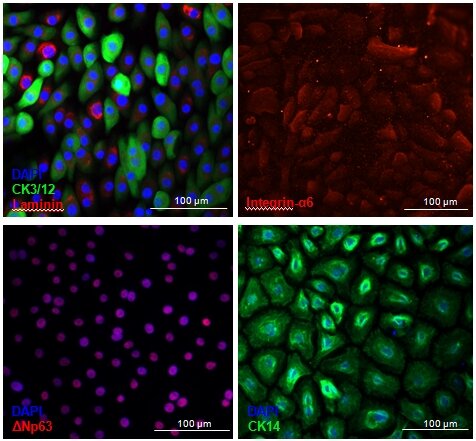
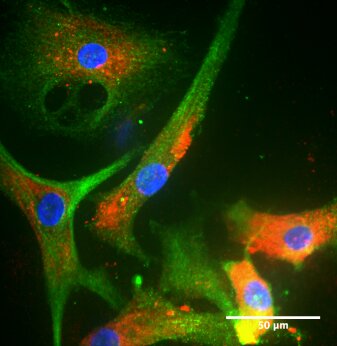
Figure 4: Cultivated LESC keep expressing phenotypical LESC-markers. ΔNp63 is a nuclear stemness marker. Integrin-α6 and laminin are extracellular matrix adhesion proteins. Cytokeratin 3/12 is a corneal epithelial marker whereas Cytokeratin 14 is a marker of Transient Amplifying Epithelial cells.
Development of a biomimetic cornea combining additive manufacturing and stem cell technologies.
Each year, there are up to two million new cases of monocular corneal blindness due to ocular trauma, corneal ulceration and a wide variety of inflammatory and infectious diseases, including 500 annual new cases in Flanders alone. More than often, not only the epithelium is damaged but also the stroma is affected, which leads to the activation of otherwise quiescent keratocytes that are transformed to fibroblasts. Corneal scars can last for decades and lead to functional blindness in the patient. However, only approximately 120,000 corneal transplants are carried out every year due to donor shortages (of which merely 350 are performed in Flanders), leaving about ten million untreated patients globally. The main reason for this discrepancy is the high shortage of suitable donor corneas.
Other limitations to the transplantation of a donor cornea include the fact that no matching is done with respect to the recipient, neither biologically nor in terms of geometry or biometry, and despite donor screening within existing cornea banks, there is always a risk of disease transmission. The limitations listed above indicate a need to develop alternatives to the use of donor corneas. The first ideas on the development of an artificial cornea date back from the early 1960’s. All currently available artificial corneas present a high risk of serious complications such as rejection, extrusion, retroprostethic membrane formation, calcification, glaucoma, retinal detachment and failure. Their use is therefore limited as a last resort to the high risk corneal transplant group of patients for which donor transplantations have previously failed.
The aim of this project is to address the need for an alternative by developping a biomimetic cornea through stem cell technologies and additive manufacturing (AM). In collaboration with KU Leuven (which focuses on AM aspect of the project) scaffolds will be 3D-printed, using human recombinant collagen (HRC) as a bio-ink and corneal mesenchymal stem cells that are isolated, characterized and optimally expanded, including their differentiation into keratocytes, within the scope of this project. A bioreactor will be developed (in collaboration with the Product Engineering Department of the UA) that provides optimized conditions in which the biocorneas can be cultured. Interactions between cells and scaffolds are studied extensively in order to allow adaptations to be made to the scaffolds, depending on the needs of the cells and vice versa. In a final step we will determine if the biocorneas are biocompatible and whether they can be integrated in vivo in the eye.
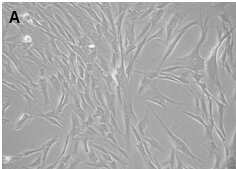
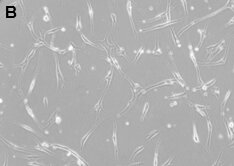
Figure: Corneal stromal Mesenchymal Stem Cells (A). Differentiated corneal keratocytes (B)
Development of a biocompatible corneal endothelial cell based therapy to address global corneal donor shortage
The corneal endothelium is the innermost layer of the cornea and has the primary function of keeping the cornea in a state of relative dehydration by pumping out excessive fluid from the cornea. A defective corneal endothelium leads to a cloudy and swollen cornea. The most common causes for this endothelial pathologies are complications during cataract surgery and some hereditary diseases, which together account for 25% of all performed corneal transplantations. Recently, a modification of the conventional therapy (penetrating keratoplasty technique or full-thickness corneal transplantation) allows partial transplantation of only this defective monolayer of cells (i.e. DS(A)EK and DMEK). These partial transplantations report good success rates and deliver better outcomes for the patient, as it is less invasive and preserving the integrity of the eye globe.
Despite its success, corneal transplantation (either full-thickness or partial) is limited worldwide by the shortage of suitable donor corneas incurring long waiting times. This global donor cornea shortage keeps increasing due to limited cornea donors coupled with stringent requirements for donation, the need for repeat surgeries and an ageing population.
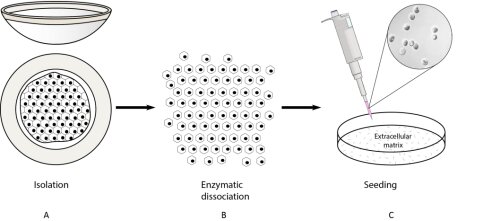
This translational project aims to investigate ex vivo expansion of corneal endothelial cells to develop a cell sheet based therapy. The principle is to expand primary human corneal endothelial cells isolated from human cadavers and to seed them on an ideal scaffolding material to introduce these cells in the patient. This concept was already proposed in 1978, but researchers have found it notoriously difficult to culture the corneal endothelial cells and as a result have not been able to implement this approach. If successful, this would change the current situation where only one donor can be used to treat one patient.
At this moment the priority in this project is the optimisation of corneal endothelial cell cultivation. Although these cells are difficult the culture, we have now a standardized protocol to expand primary endothelial cells isolated from human cadaveric corneas (figure). During the optimisation of these cultivation processes there is an extensive characterisation of these cells going on to insure their specific morphology, pheno- and genotype. The next step in the future is to investigate and characterize the ideal cell scaffolds to introduce these characterized cells in a rabbit model.