First infants and pregnant women recruited in mpox vaccine trial in the DRCongo
The PregInPoxVac project marks a key milestone and launches official project website
Boende, Democratic Republic of the Congo — The PregInPoxVac research project, an international clinical trial aiming to protect pregnant women, new mothers, and children under two from mpox (previously known as monkeypox), has vaccinated the first infants and recruited the first pregnant women in Boende, a region at the centre of the ongoing mpox outbreak in the Democratic Republic of Congo’ (DRC).’
In August 2024, the ongoing multi-country outbreak of mpox was declared a continental emergency by Africa CDC and a Public Health Emergency of International Concern by the World Health Organization. Since 2024, in the DRC alone, the outbreak has led to over 22,000 confirmed cases, with severe symptoms including bronchopneumonia, sepsis, encephalitis, and even death.’ However, the suspected cases are much higher - especially among children. Pregnant women are also at increased risk of severe complications from mpox. Despite this, they and their infants have not been eligible for vaccination.
-
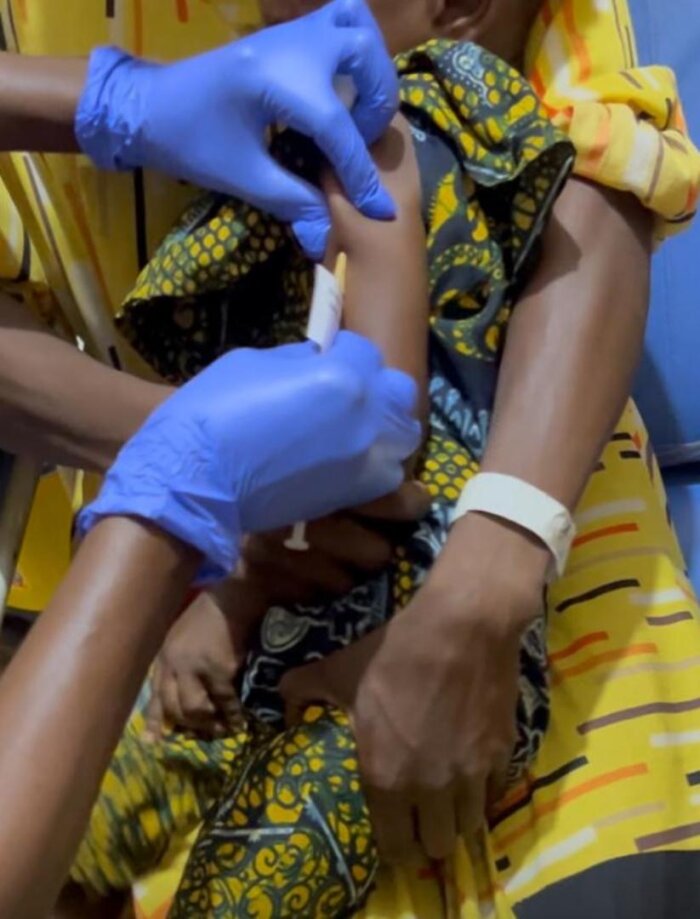 First vaccinated infant
First vaccinated infant -
 Service de maternité
Service de maternité -
 Workshop
Workshop
The PregInPoxVac project is a first-of-its kind study which aims to address this critical evidence gap. “Mass vaccination campaigns are hampered by the fact that the mpox vaccine has not been approved in infants & Pregnancy, these study results will aid to make these vaccine campaigns really comprehensive and impactful” says Jean-Pierre Van geertruyden, prof. Global Health & Disease control.
Led by the University of Antwerp (Belgium) and the University of Kinshasa (DRC), and supported by partners in Kenya (ACE Research) and Italy (Penta Foundation), the study is evaluating the safety and immunogenicity of the MVA-BN® (Jynneos) vaccine for the first time in two highly vulnerable groups: pregnant and postpartum women and infants aged 4 to 24 months. The project is funded by the European Union and the Coalition for Epidemic Preparedness Innovations (CEPI) and Global Health EDCTP3, with support from Bavarian Nordic, the manufacturer of the MVA-BN® vaccine.
“Research indicates that pregnant women are at an increased risk of disease-related complications from mpox, and infants are more likely to experience severe mpox symptoms” said Dr Kristine Rose, CEPI’s Mpox Disease Programme Leader. “Such findings underscore the importance of evaluating vaccine safety and immune responses to enable protection of these vulnerable populations. Global attention to mpox is urgently needed as the virus continues to ravage countries across Africa and the world, with particularly explosive outbreaks ongoing in the DRC and Sierra Leone showing no signs of stopping.”
MVA-BN® is already approved for adolescents (12 years and older) and adults in several countries. The MVA-BN-Filo® vaccine, an Ebola vaccine using the same base as the MVA-BN® vaccine, has shown a good safety profile in pregnant women, making the MVA-BN® vaccine a promising candidate for protecting these at-risk populations. Over the coming months, the trial will enrol 215 pregnant, 144 post-partum women and 344 infants. All participants will be closely monitored for side effects and immune response. The ultimate goal is to generate robust data to guide future vaccination policies and ensure that pregnant women and infants are no longer left behind in mpox outbreaks.
“We are proud to have vaccinated the first children in this important trial. A significant milestone for maternal and child health in the fight against mpox. Although these populations are vulnerable to the mpox infection, they are often excluded in clinical trials”, says Hypolite Muhindo Mavoko, prof. at the Department of Tropical Medicine at the University of Kinshasa.
Paul Chaplin, President and Chief Executive Officer of Bavarian Nordic, said: “We are proud to support this project which aims to bridge the gap for access to protection against mpox for the most vulnerable populations. Through partnerships we have made significant advances already by expanding access to our mpox vaccine for children and adolescents, and we applaud the collaborators and funding partners for enabling this important study, that will generate evidence on the use of MVA-BN in infants and pregnant women.”
Data from the trial will be published in open-access journals so that it can be readily available to the entire public health community to inform current outbreak response efforts and future research.
In parallel, the project combines clinical research with a social science study (led by the Penta Foundation) to explore the acceptability and perceptions of participants and the wider community regarding mpox, the vaccine and the ongoing trial. Ancillary care services are also being provided to support participants’ broader health needs during the study. Additionally, mpox surveillance systems are being strengthened in collaboration with local health authorities to improve disease tracking and enable early response.
Funding for the project was first announced in November 2024.