Spectro-electrochemistry
Spectroelectrochemistry can provide unique insights into interfacial reactions and redox processes. In a typical example, in situ UV-VIS absorption spectroscopy follows a redox conversion at an optically transparent or metallic grid electrode. This allows to identify participating reaction species and determine the energy and mechanism of redox conversions, basically answering “What?” and “How?”.
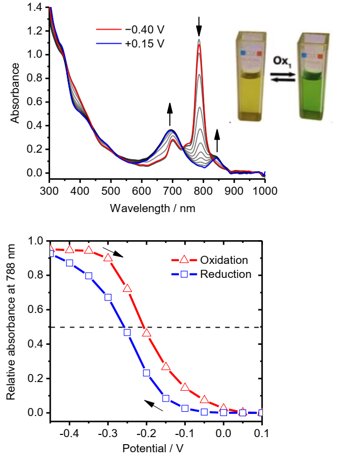
Spectroelectrochemical study of Zn naphthalocyanine confirms reversible one-electron transition associated with the oxidation of the macrocycle around -0.23 V, whereas voltammetric behavior was unclear due to strong aggregation.