Inflammasomes
The team of Andy Wullaert investigates the function of cytosolic protein complexes termed inflammasomes that play important roles in innate immune responses. Figure 1 shows how inflammasome activation happens through a sensor protein that senses the trigger, after which the protease caspase-1 and the pore-forming protein GSDMD mediate a lytic cell death termed pyroptosis that releases the IL-1β and IL-18 cytokines exerting inflammatory effects. We study inflammasomes in infectious diseases, in autoinflammatory diseases, and in humanized mouse models.
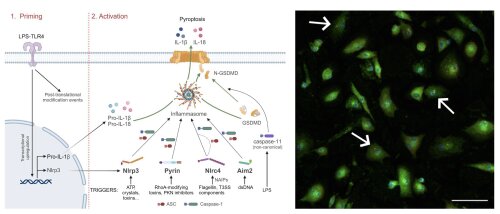
Fig 1: Simplified scheme of the two-step process for activation of various inflammasomes (left) and immunostaining for activated inflammasomes in Nlrp3-activated primary mouse macrophages (right). A first priming step leads to transcriptional upregulation of inflammasome components. A second, activation step activates specific Nlrp3, Pyrin, Nlrc4 or Aim2 inflammasomes, which are assembled in ASC specks (red dots in right panel) in which caspase-1 is activated. Caspase-1 (or -11 in non-canonical inflammasome activation) cleaves gasdermin D (GSDMD) as well as the pro-forms of IL-1β and IL-18. These matured IL-1β and IL-18 cytokines are then released through pyroptosis. Created with BioRender, reproduced from the PhD dissertation of Giulia Doglio.
Inflammasomes in infectious diseases
Depending on the sensor protein, various inflammasomes can be discriminated that react to different triggers. These triggers often are of microbial origin, relating to important host-protective effects of inflammasomes during infections. Studying inflammasome signaling during infections is one of the focus areas of the research team. For instance, we revealed some of the mechanisms by which Nlrp3 inflammasome activation protects against bacterial (Mamantopoulos et al. 2019), fungal (Rogiers et al. 2019) and viral (Dubois et al. 2019) pathogens. More recently, we expanded our work towards studying how cooperation between different cell death modes protects mice against intestinal Citrobacter rodentium infections (Eeckhout et al. 2023).
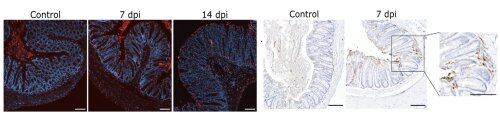
Fig 2: TUNEL staining demonstrating cell death (left) and immunostaining for cleaved caspase-8 indicating apoptotic cell death (right) in colon of Citrobacter rodentium infected mice. Reproduced from Eeckhout et al. 2023.
Inflammasomes in autoinflammatory diseases
The evolutionary battle between humans and pathogens turned the beneficial inflammatory functions of inflammasomes in host defense towards deleterious inflammatory effects in people with gain-of-function mutations in genes encoding inflammasome components. These people suffer from so-called ‘inflammasomopathies’, which are a group of rare auto-inflammatory diseases (AIDs) characterised by flares of spiking fever and inflammation. Studying the molecular and cellular mechanisms underlying these inflammasomopathies is another focus area of the team. For this purpose, we use genetic mouse models as well as genome-edited human cell lines to mimic human AIDs, and primary PBMCs from AID patients. For instance, we reported on the role of macrophages during cryopyrin-associated periodic syndromes (CAPS) using a genetic mouse model with macrophage-specific expression of a hyperactive Nlrp3 inflammasome (Frising et al. 2022), and we generated a genetic mouse model for an auto-inflammatory disease caused by NLRC4 hyperactivation (Eeckhout et al. 2023). In addition, we investigate less obvious involvements of inflammasomes in other types of AIDs, such as the involvement for the Nlrp3 inflammasome in OTULIN-related auto-inflammatory syndromes (Doglio et al. 2023).
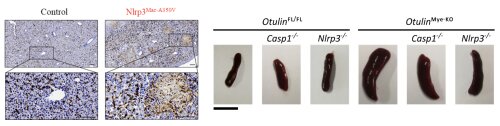
Fig 3: Immunostaining showing macrophage infiltration in livers of mice expressing a hyperactive Nlrp3 inflammasome (left, from Frising et al. 2022), and spleen pictures showing reduced splenomegaly when deleting the Nlrp3 inflammasome in mice lacking Otulin in myeloid cells (right, from Doglio et al. 2023).
Inflammasomes and innate immunity in humanized mouse models
Innate immunity pathways, and inflammasome signaling in particular, are very different in humans when compared to mice. These species-specific differences hamper the translation of experimental findings in mice towards clinical applications in humans. For this reason, our team also focuses on establishing humanized mouse models to experimentally study human innate immune responses in an in vivo setting. Because myeloid cells that execute innate immune responses engraft poorly in traditional immunodeficient mice, we use immunodeficient mice with transgenic expression of human myelopoiesis promoting factors. In this respect, we recently validated the efficient engraftment as well as the functionality of human myeloid cells in so-called NSG-QUAD mice. We also showed that humanized NSG-QUAD mice can be used for in vivo pre-clinical testing of small molecules targeting human innate immune pathways (Stocks et al, 2024). We are further characterizing and validating this humanized mouse model for more reliably studying the various innate immune mechanisms underlying human inflammatory diseases.
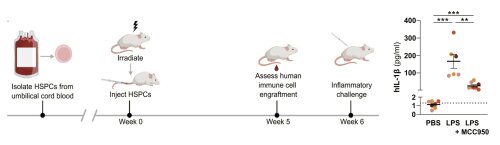
Fig 4: Schematic overview of the NSG-QUAD humanization and analysis procedures (left), and proof-of-concept that the efficacy of a small molecule (MCC950) to block human-specific inflammasome-generated cytokine responses can be assessed in vivo in humanized NSG-QUAD mice (right). Figures reproduced from Stocks et al, 2024