Self-Association
Solutions in liquefied inert gases have proven to be an ideal medium to study molecular complexes held together by weak and medium-strong interactions. They create a weakly interacting environment which, combined with the low temperatures used, leads to small bandwidths and thus facilitates the detection of complex bands only slightly shifted from the monomer modes. Although most experiments reported are focusing on hetero complexes, involving an electron rich Lewis base and an electron deficient region related to a hydrogen bond, a halogen bond or a lone pair-pi acceptor, these solutions also form an ideal medium to investigate self-association through hydrogen bonds between two, three, or more identical molecules.
Self-associating molecules have a structure which provides a hydrogen bond donor group and an acceptor group in one molecule, and can self-associate trough one or more hydrogen bonds. These kinds of species often serve as easily accessible prototypes to investigate a variety of hydrogen bonding interactions.
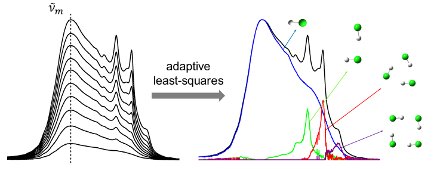
In order to get more information on the self-associating behavior in cryosolutions, we developed and validated numerical methods in which the concentration dependent behavior observed for the dissolved molecules is analyzed by least‑squares fitting approaches. In these methods, for each wavenumber a polynomial is used to mimic the relation between monomer concentrations and measured absorbances. It was shown that by carefully analyzing the differences between consecutive fittings and by introducing additional constraints, the contributions due to monomers and self‑associated species can be determined with a much higher accuracy than before. This way overlapping absorption bands originating from monomers and oligomers can be resolved and integrated to get more information on the bonding enthalpies.