Why van Gogh's Sunflowers are wilting
The colour of Vincent van Gogh's famous Sunflowers is changing over time, because of the mixture of pigments used by the Dutch master in his painting. X-ray examination shows how chrome yellow darkens.

Sunflowers, 1889, Vincent van Gogh (1853-1890). Credit: Van Gogh Museum, Amsterdam (Vincent van Gogh Foundation)
Evidence for the process now comes from a detailed spectroscopic investigation of the Sunflowers version at the Van Gogh Museum in Amsterdam. A group of scientists headed by Letizia Monico from the Institute of Molecular Science and Technology (CNR-ISTM) of Perugia, the University of Perugia and the University of Antwerp, shone X-rays from DESY’s lightsource PETRA III through tiny particles of paint taken from the painting.
They describe their results in the journal Angewandte Chemie International Edition. The study also identifies areas of the painting that should be monitored particularly closely for any changes.
Vincent van Gogh (1853-1890) is famous for his use of bright yellow colours. The Dutch painter used so-called chrome yellows, a class of compounds consisting of lead, chromium and oxygen. “There are different shades of the pigment, and not all of them are photochemically stable over time,” explains Monico. “Lighter chrome yellow has sulphur mixed into it, and is susceptible to chemical degradation when exposed to light, which leads to a darkening of the pigment.” Lightfast chrome yellow has the chemical formula PbCrO4, whereas the light-sensitive type has the formula PbCr1-xSxO4, (with x exceeding about 0,4).
The scientists examined a Sunflowers painting, which dates back to 1889, to determine whether van Gogh had used different types of chrome yellow when painting it. He produced three versions of the painting, one of which is on display at the National Gallery in London, one at the Seji Togo Memorial Sompo Japan Nipponkoa Museum of Art in Tokyo and one at the Van Gogh Museum in Amsterdam. Two small paint samples, measuring less than 1 millimetre each, were taken from the painting in Amsterdam and examined using DESY’s X-ray source PETRA III. “The analysis shows that the orange-yellow hues mainly contain the lightfast version of chrome yellow, whereas the light-sensitive type is mainly found in the pale yellow areas,” reports co-author Gerald Falkenberg, who is in charge of DESY’s beamline P06, where the X-ray diffraction measurements were carried out.
At the European Synchrotron Radiation Facility (ESRF) in Grenoble, the team examined the chemical state of the paint samples. When light sensitive chrome yellow darkens, the chromium is reduced from its highest oxidation state CrVI to CrIII. The scientists were indeed able to detect a relative proportion of 35 per cent CrIII on the surface of the paint. “At least at the two sites from which the paint samples were taken, a colour change has occurred in the Sunflowers as a result of the reduction of chrome yellow,” says Monico. This suggests that the Sunflowers may originally have looked different from what we see today.
The scientists used a mobile scanner to identify those parts of the painting which ought to be monitored particularly closely for possible changes. “Since chrome yellow pigments were widely used by late 19th-century painters, this study also has broader implications for assessing the colours of other works of art,” emphasises co-author Koen Janssens, from the University of Antwerp.
Media articles related to this research
- Van Goghs geel verschiet van kleur - De Standaard, 23 October 2015
- Why van Gogh's Sunflowers are wilting - Eurekalert, 22 October 2015
- Van Gogh's 'Sunflowers' wilting - The Times of India, 22 October 2015
- Van Gogh's Sunflowers painting wilting - Deccan Heralf, 21 October 2015
- Zonnebloemen doorgelicht - C2W, 21 October 2015
- Most powerful X-ray shows why Van Gogh’s sunflowers are fading - RedOrbit, 21 October 2015
Van Gogh’s pigment up close: Fading Orange-Red

Red lead is most familiar to us in orange-red rustproof paint. Artists have treasured the brilliant color of this pigment for their paintings since ancient times. However, various ageing processes cause discoloration of the saturated hue over time. Thanks to a combination of X-ray diffraction mapping and tomography experiments, a team headed by Koen Janssens at the AXES research group has now explained an additional step in the light-induced degradation of lead red. The key to their discovery was the identification of the very rare lead carbonate mineral plumbonacrite in a painting by Van Gogh, as the researchers report in the journal Angewandte Chemie.
“This is the first time that this substance has been found in a painting from before the mid twentieth century,” reports Frederik Vanmeert, first author of the paper. “Our discovery sheds new light on the bleaching process of red lead.”
You can find out more about this research and publication in the press release below.
Blackening of the red pigment vermillion
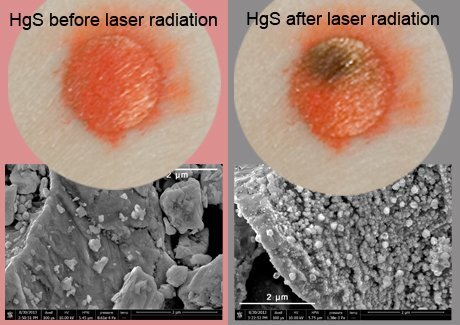
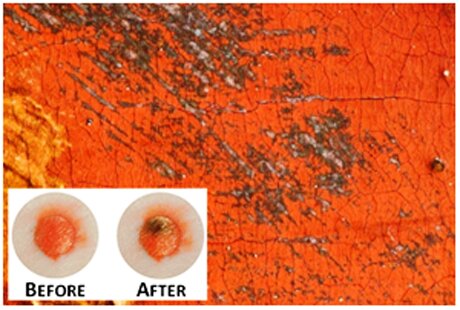
For centuries on end, vermillion red remained one of the most popular pigments and was used by our greatest painters. It is a widely known fact, however, that over time the red degrades and turns black. Researchers at the University of Antwerp have finally been able to pinpoint the cause of this degradation: mercury. Their research has been published in the prestigious journal Angewandte Chemie.
Media articles related to this research
- Rood op Rubens' schilderijen verkleurt naar zwart - Vandaag.be, 22-09-2011
- Kwik laat vermiljoenrood verkleuren in meesterwerken - Het Laatste Nieuws, 07-10-2013
- Paintings turning black? Blame mercury - Nature, 04-10-2013
- Kwik is boosdoener bij verkleuring schilderijen - De redactie, 07-10-2013
- Zeezout bedreigt Rubens - Gazet van Antwerpen (online / gedrukt), 27-09-2014
Press release: Mercury tears cause the discoloration of paintings
Date: 8 oktober 2013
Introduction: Scientists at the University of Antwerp make a breakthrough and solve a key problem in art history.
For centuries on end, vermillion red remained one of the most popular pigments and was used by our greatest painters. It is a widely known fact, however, that over time the red degrades and turns black. Researchers at the University of Antwerp have finally been able to pinpoint the cause of this degradation: mercury. Their research has been published in the prestigious journal Angewandte Chemie.
Ancient Roman frescos in Pompeii and the masterpieces of Rubens, Peter Brueghel and many other celebrated artists: all have deteriorated as the vermillion pigment used in the paint turns black. Scientists had already ascertained that this is caused by exposure to light, chlorine and moisture but the substance which triggered the chemical reaction remained a mystery. Antwerp researchers have recently made a breakthrough, however.
“Several hypotheses had already been suggested”, says project manager Professor Karolien De Wael (University of Antwerp). “Conducting a number of electro-chemical experiments has now enabled us to establish exactly what happens to the vermillion.”
The scientists applied pigment to an electrode and exposed it to light and chlorine ions. An electric voltage was then passed through the electrode and the current measured.
"When the pigment was exposed to light and chlorine ions we were able to detect an increased current at a particular voltage”, explains De Wael. “What’s more, the voltage required turned out to be exactly that needed for metallic mercury. In effect, then, you could say that paintings such as The Adoration of the Magi by Peter Paul Rubens, which belongs to the collection of Antwerp’s Royal Museum of Fine Arts, are weeping mercury tears. We used paint samples from this masterpiece for our research.”
Volatile mercury
“It wasn’t easy to prove that metallic mercury is being produced because it tends to be very volatile”, admits Professor Koen Janssens (UAntwerp). “It forms but before you have the chance to analyse it, it evaporates or reacts again.” The mercury accumulates in the ‘pores’ and in the many crevices in the paint, ultimately leading to the discoloration of the vermillion.
The results of the Antwerp study have not gone unnoticed. The latest issue of prestigious journal Angewandte Chemie* includes an article about the research, which makes sense since this discovery may facilitate better conservation of historic masterpieces. “This discoloration is usually influenced by light and chlorine”, explains Willemien Anaf, who is a PhD student. “Of course, it is impossible to store paintings in the dark but museums face an important challenge in keeping chlorine away from the paint. Protective layers of varnish play an important role here.”
The News pages of leading scientific journal Nature have also reported on the study’s findings.