The platform significantly shortens the process for testing drug candidates and biomarkers by a factor of 5 compared to research using animal models, taking only four months compared to over more than a year (Fig 1).
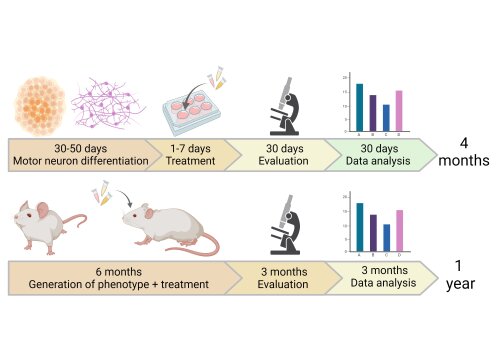
Fig 1. A schematic representation of the time it takes to test a therapeutic compound using SCREEN4PN iPSC technology, in comparison to conventional mouse model studies. (Created with BioRender.com)
Other benefits provided by SCREEN4PN include:
- Improved standardisation: With iPSCs becoming increasingly more valuable as a disease model, SCREEN4PN aims to standardize the protocols used to generate cell types related to peripheral neuropathies, reducing variability. As we improve the standardization of the human IPSC model, we increase our reproducibility leading to more reliable results.
- SCREEN4PN is more versatile and expandable than animal models are. We intend to expand the platform to cover more peripheral neuropathies, and improve our mimicry of the human physiological complexity.
- Due to SCREEN4PN using human cells, we are capable of better simulating human pathology than animal models. The cells have a human genetic background, and when derived from patients, already contain the mutation needed to model the disease (Fig 2).
- Any type of drug or biomarker can be tested on SCREEN4PN.
- SCREEN4PN has significantly reduced costs in comparison to mouse model research.
- The versatility of the platform, paired with optimized and efficient read-out methods, leads to easy validation of any results obtained.
- SCREEN4PN respects the 3R's principle of animal model research, in particular with the "Reduction" aspect.
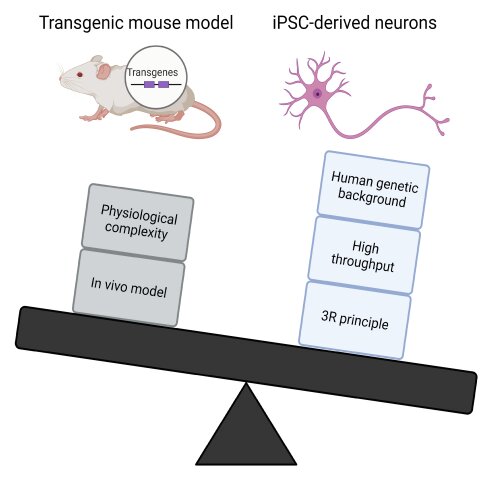
Fig 2. SCREEN4PN "outweighs" conventional mouse model research by more than time needed alone.