13/12/2022 - Charles (ESR #9)
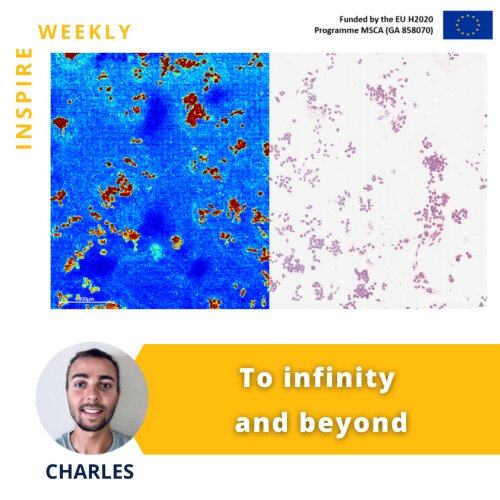
The image on the flyer shows the distribution of a specific lipid (highlighted in red) in immune cells (macrophages) using Mass Spectrometry Imaging (MSI). The left image shows the MSI image, whereas the right part shows an image acquired by light microscopy. Since its discovery more than 30 years ago, MSI scientists have been trying to push the boundaries of this technology. One major challenge of MSI has been the limited spatial resolution, i.e. the minimum length scale of an acquired image. However, in recent years, more powerful MSI instruments have been developed that allow users to image single cells and reach even subcellular resolution.
Together with my colleague Brigitta Szabó (ESR #1), PhD fellow at Ncardia, we will optimize a protocol to evaluate Doxorubicin distribution at a subcellular level in order to better understand the molecular mechanisms of this drug.
16/11/2022 - Charles (ESR #9)
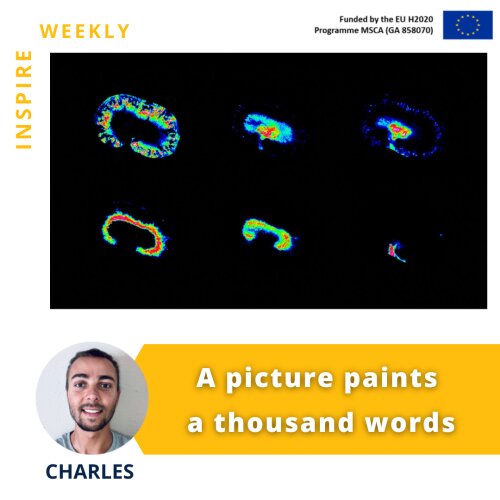
The provided image shows how mass spectrometry imaging can highlight specific metabolites distribution within the tissue (kidney), characterizing its different substructures: cortex (top left), medulla (top right), pelvis (bottom right), and vessels.
Mass spectrometry imaging (MSI) is a powerful technique that enables untargeted investigations into the spatial distribution of molecular species in a variety of samples. It can image thousands of molecules, such as lipids, peptides, proteins, metabolites, and glycans, in a single experiment without any labeling.
Together with my colleague Dustin Krüger ESR#14 from Antwerp University, we started to develop an MSI-related protocol called derivatization, in order to sensitively increase doxorubicin detection within organs like kidney (flyer image), heart, and liver. This study will allow us to evaluate doxorubicin-induced toxicity and will lead to a better understanding of the molecular mechanisms in the organs that metabolize the drug.
02/11/2022 - Haibo (ESR #3)

Drug-related cardiotoxicity (i.e., any heart damage arising from drug side effects) is important in drug development [1]. It is crucial for the pharmaceutical industry to develop effective methods to identify the cardiotoxicity risk in drug development at an early stage. In the early stage of drug development, pharmaceutical companies often use Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes to test the drug safety and record some field potential signals (extracellular electrical activities) of those cells. By analyzing those field potentials, pharmaceutical companies can study the drug effects on those cells. However, analyzing those filed potential signals is laborious because the size of those signals is large. So, computer-based automatic analysis tools can empower the analysis of drug-related cardiotoxicity risk.
Therefore, in INSPIRE’s recent publication by Haibo Liu, we shared the results of using several Artificial Neural Network methods to predict drug adverse effects on cardiomyocytes. Moreover, this study tested the abilities of those Artificial Neural Network methods to automatically detect if a drug is safe or not. It will help to improve the efficiency of drug development by detecting cardiotoxicity in a more fast and effective way. Please check the article to find out more details:
Haibo Liu, Tessa De Korte, Sylvain Bernasconi, Christophe Bleunven, Damiano Lombardi, Muriel Boulakia. Artificial Neural Network Comparison on hERG Channel Blockade Detection. International Journal Of Computer Applications. Volume 184 - No.14, May 2022. DOI: 10.5120/ijca2022922119. Open Access
Reference:
- Nicola Ferri et al. “Drug attrition during pre-clinical and clinical development: Understanding and managing druginduced cardiotoxicity”. In: Pharmacology and Therapeutics 138.3 (2013), pp. 470–484. ISSN: 0163-7258. DOI: https://doi.org/10.1016/j.pharmthera.2013.03.005.
17/08/2022 - Anna (ESR #15)
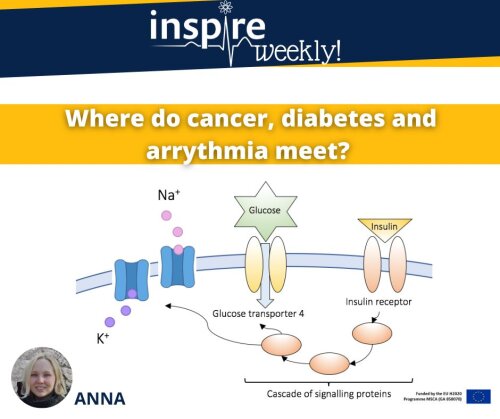
In safety pharmacology, we aim to increase the safety of drugs and reduce their side effects.
Specifically, I am working on arrhythmia as a side effect of anti-cancer drugs.
Anti-cancer drugs termed Receptor Tyrosine Kinase Inhibitors (RTKIs) target protein receptors on the cell membrane. RTKIs also have the effect of inhibiting the insulin signaling pathway, which is something that naturally occurs in diabetic patients.
Since both diabetic and oncology patients develop arrhythmias, the insulin pathway is considered the main suspect!
The arrhythmia in these people is given in part by the altered activity of ion channels present on the cell membrane. When ions flow through them, they cause a cardiac cell to contract or to relax, so their activity determines with which frequency the cells "beat"!
How do we analyze ion channels' activity? Patch clamp!
This electrophysiology method allows recording ion flows within a single cell, like hiPSC-CMs which are my model of choice.
Overall, I am studying how long-term drug treatment, including RTKIs, affects the activity of ion channels. Discovering these mechanisms could prevent drugs under development from causing arrhythmias!
References
- D. Glovaci, W. Fan, N. D. Wong, Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr Cardiol Rep 21, 21 (2019).
- J. E. Salem et al., Anticancer drug-induced life-threatening ventricular arrhythmias: a World Health Organization pharmacovigilance study. Eur Heart J 42, 3915-3928 (2021).
20/04/2022 - Benji (ESR #13)
Cancer is one of the leading causes of morbidity and mortality. In 2020, the worldwide incidence of cancer was estimated at 19.3 million and the mortality at 10 million.[1] Thus, many scientific studies focus on understanding cancer biology and pathophysiology, on finding new and better treatments, and on early diagnosis and prevention of cancer. Between 2006 and 2018, annual investments in cancer research increased from 5.6 billion to 8.5 billion by the international cancer research partnership (ICRP), a worldwide network of cancer funding organizations.[2] On average the development of a new treatment, from early development to marketing, costs over 1 billion per medicine over a period of roughly 20 years; this development includes safety as well as efficacy testing.
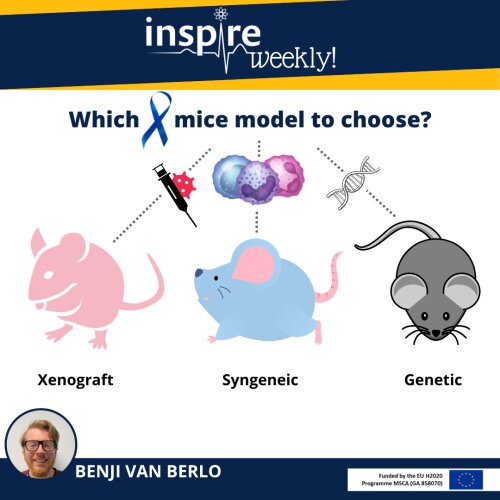
Testing the functionality and safety of new treatments is performed in several stages starting with in vitro testing e.g. using cancer cell lines for efficacy testing and healthy human cell lines from different organs to assess toxicity. In the next stage, laboratory animals such as mice are used to investigate the efficacy of the drug in vivo. Different cancer mice models can be used to test drug efficacy all having advantages as well as disadvantages. Here, we will describe three frequently used models.
1) The xenograft mice model: These are nude mice without an immune system, which allows for injection of human cancer cells without rejection by the immune system and thus tumor development. The advantage of this model is that it is easy in use and human cancer cells can be used, thus more closely mimicking the human condition. The main disadvantage of this model is the absence of the immune system, which is known to play an important role in cancer and which leads to specific housing requirements of these mice.
2) Syngeneic mice model: In a syngeneic mice model, inbred mice are used. These are mice that are genetically identical to each other as a way to minimize variation in scientific studies caused by difference in genetic background. In rare cases, these mice develop cancer spontaneously or as a result of chemical treatment. When this happens these cancer cells can be isolated and cultured allowing continued growth of these cancer cells ex vivo in a laboratory flask. Because the cells are genetically identical to the host, re-introducing the cells into a host with a similar genetic background is possible, leading to the formation of a tumor. Some of these syngeneic cancer cell lines are already in use for several decades. The main advantage of this model compared to the xenograft model is the presence of an intact immune system. However, since these cells where isolated from mouse tumors, the human condition is less closely mimicked due to potential differences between species.
3) Genetic mice model: Perhaps the best translational mice model to humans, genetic mice models have a specific mutation(s) in their DNA leading to spontaneous development of cancer. Often, the DNA mutations induced in these mice are similar to the human mutation in cancer patients, thereby more closely mimicking the human condition. The main disadvantages of this type of model is that they are more expensive compared to e.g. the syngeneic model and studies using genetic models often take longer due to the high latency time of cancer development.
All three mice models are frequently used in scientific studies to investigate the safety and efficacy of new medicines, but also to unravel the tumor biology or assess cancer enhancing properties of substances or proteins. The same models can also be used to investigate the shared pathophysiology between diseases, for example cardiovascular diseases and cancer. Recent studies using these mice models have demonstrated enhanced cancer growth in the presence of a cardiovascular disease, specifically heart failure.
The focus of my PhD project is to unravel the molecular mechanisms leading to enhance cancer growth in the presence of heart failure specifically focusing on endothelial growth factors.
All in all these mice models provide scientist with powerful tools for cancer research.
References:
- Ferlay, J., et al., Cancer statistics for the year 2020: An overview. International Journal of Cancer, 2021. 149(4): p. 778-789.
- Abudu, R., et al., Trends in International Cancer Research Investment 2006-2018. JCO Global Oncology, 2021. 7: p. 602-610.
13/04/2022 - Christian (ESR #4)

Understanding animal behaviour is crucial to a large variety of research fields. Recent computational and technological advances have led to several automated methods and technologies to measure and quantify behavioural aspects of a wide variety of animal species, ranging from basic voluntary locomotor activity towards a more complex and comprehensive behavioural repertoire. These advances also enhance the opportunity to measure animals´ behaviour in their most ethological meaningful laboratory environment: the home-cage.1
As part of my PhD I reviewed existing methods and technologies which are frequently applied to measure behavioural aspects of rodents in their home-cage.2 These range from targeting voluntary locomotor activity measurements (electrical capacitance technology and RFID) towards more advanced methods which expand the analysis of the behavioural repertoire beyond basic locomotor by force plates, infrared beam frames or (RFID-assisted) video tracking technology. It also sheds light on their benefits and limitations and aims to support (non-)behavioural and multidisciplinary researchers in choosing an appropriate method for their specific research needs, especially for those who accepted the challenge to introduce social housing as a refinement strategy and thereby contributing to the 3Rs. It furthermore gives insights into current and future technological developments emphasising on the challenges associated with monitoring multiple rodents in a social context.
References:
- Voikar, V., & Gaburro, S. (2020). Three pillars of automated home-cage phenotyping of mice: novel findings, refinement, and reproducibility based on literature and experience. Frontiers in Behavioral Neuroscience, 193.
- Klein, C. J. M. I., Budiman, T., Homberg, J. R., Verma, D., Keijer, J., & Van Schothorst, E. M. Measuring locomotor activity and behavioral aspects of rodents living in the home-cage. Frontiers in Behavioral Neuroscience, 115.
30/03/2022 - Martina (ESR #2)
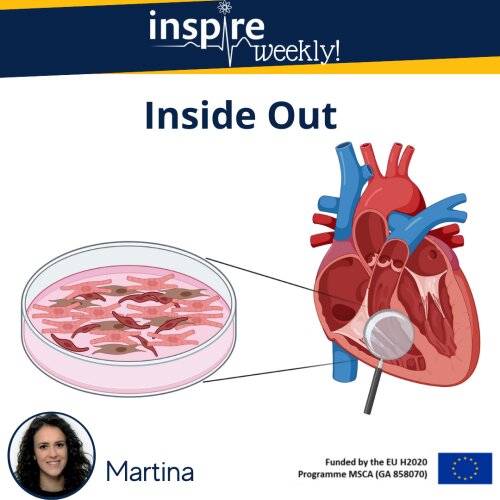
Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) are a well-established model for cardiac toxicity testing. However, this model does not consider other cellular types present within the cardiac tissue. The human heart environment comprises several cell populations: cardiomyocytes, fibroblasts, endothelial cells, and other type of cells. During the last decade, 3D co-culture of cardiac micro-tissues has gone more and more under the spotlight as a novel approach to improve hiPSC-CM maturation and obtain more physiologically relevant results. Even if the employment of 3D cultures is impeded by several challenges and technical difficulties, so far different studies have shown promising results of cardiomyocytes response to drugs, resulting in a more predictive approach [1] [2].
During my PhD, I will investigate the 2D co-culture of hiPSC-CMs, endothelial cells and fibroblasts in a 96-well format on Real Time Cell Analyzer (RTCA) Cardio Extra-Cellular Recording (ECR), to evaluate the improvement of cardiomyocytes maturity and compare the differences in terms of drug cardiotoxicity predictivity between the mono- and co-cultures through the analysis of functional parameters, such as impedance, contractility, and electrophysiology.
References:
- FUJIFILM Cellular Dynamics, Inc. (FCDI). 3D Tri-culture Cardiac Microtissue Assays with Simplified Workflow and Off-the-Shelf Reagents. iCell® Cardiomyocytes Application Protocol.
- Sirenko O, Hancock MK, Crittenden C, Hammer M, Keating S, Carlson CB, Chandy G. Phenotypic Assays for Characterizing Compound Effects on Induced Pluripotent Stem Cell-Derived Cardiac Spheroids. Assay Drug Dev Technol. 2017 Aug/Sep;15(6):280-296. doi: 10.1089/adt.2017.792. PMID: 28837356.
16/03/2022 - Marieke (ESR #11)

The cells in the picture are HEK293 cells, usually 20 – 30 µm long, here seen with the microscope at 10x magnification. HEK293 cells were isolated from a human embryonic kidney for the first time in the Netherlands in 1973, so almost 50 years ago. Today, they are still used in laboratories all around the world. The HEK293 cell line is an immortalised cell line, meaning they don’t die, but keep reproducing and can survive in an artificial cell culture environment.1 The cell line is very popular thanks to its relatively easy growth and maintenance in cell culture and its ability to express recombinant proteins (e.g., ion channels or receptors on the cell surface).1,2 Several variations on the cells line are available. One example are the HEK293T cells, expressing the large T antigen and displaying an even more efficient transfection.1,2
I’m using this HEK293T cell line in my PhD project to express the VEGFR2, a receptor known to be involved in development of hypertension, linked to a luciferase. The day before the experiment, a DNA construct, containing the receptor and the luciferase, is added to the cells so that the next day, they will express this receptor-luciferase on their membrane and the experiment can be carried out, looking at how drugs are interacting with VEGFR2.
References:
- Thomas P, Smart TG. HEK293 cell line: A vehicle for the expression of recombinant proteins. J Pharmacol Toxicol Methods 2005; 51: 187–200.
- Arena TA, Harms PD, Wong AW. High throughput transfection of HEK293 cells for transient protein production. Methods Mol Biol 2018; 1850: 179–187.
09/03/2022 - Callan (ESR #10)
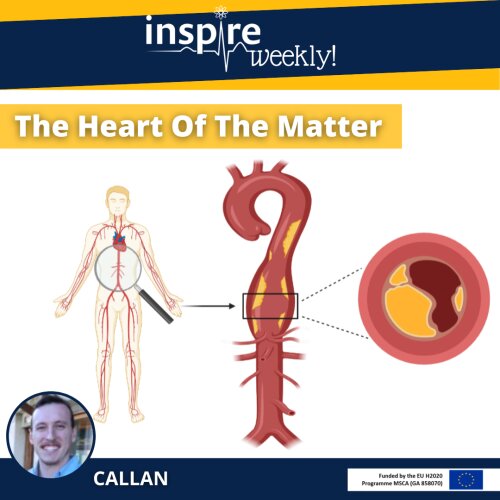
Atherosclerosis is the underlying cause of many cardiovascular diseases. Atherosclerosis is characterized by the buildup of fatty deposits, known as plaques, within the arterial walls of the large arteries (as indicated on the two right images). Normal healthy arteries are flexible and elastic in nature allowing unobstructed blood flow. However, the formation of plaques lead to the arteries becoming progressively narrower and less compliant. The implications of these actions are severe, as occlusion to the blood flow is worsened with continuation of plaque development. The most important clinical complication arises when a plaque becomes unstable, leading to the formation of a blood clot or thrombus due to an acute occlusion in the artery (Lusis 2000). This can be life threatening as it results in either a myocardial infarction or stroke. Atherosclerosis is one of the leading causes of death worldwide (Pattarabanjird, Li et al. 2021). Therefore, indicating the importance of studying such a disease and the pathological processes compounds/drugs may have on the cardiovascular system. To this end, researchers use a number of genetically engineered mouse models (e.g. apolipoprotein E or LDL receptor knockout mice) that mimic various aspects of human plaques.
Animal models mimicking human diseases are implemented in order to understand the potential efficacy and mode of action of novel pharmaceuticals. This is monumental in safety risk compounds pose in clinical and non-clinical studies helping to gain insight and understanding of the mechanism of a particular toxicity.
References:
- Lusis, A. J. (2000). "Atherosclerosis." Nature 407(6801): 233-241.
- Pattarabanjird, T., et al. (2021). "B Cells in Atherosclerosis: Mechanisms and Potential Clinical Applications." JACC Basic Transl Sci 6(6): 546-563.
02/03/2022 - Sara (ESR #8)
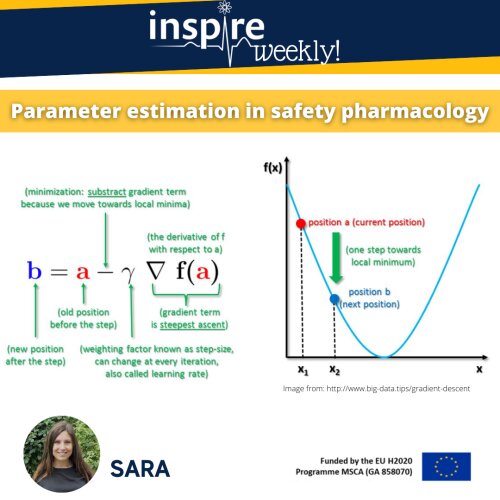
Machine learning is a form of artificial intelligence, which means the system learns from data rather than through explicit programming. Almost every machine learning algorithm has an optimization algorithm at it’s core. In this post, I will illustrate and explain a simple optimization algorithm: the “gradient descent” algorithm is frequently used to find values of parameters that cannot be calculated analytically and that have to be estimated through an algorithm.
Technically, this algorithm aims to minimize a given mathematical function (say cost function) through a repetitive stepwise approach, involving following iterative steps:
- Compute the first order derivative of the function at the current point (gradient)
- Make a step in the direction opposite to the gradient: opposite direction of slope increase from the current point by gamma (learning rate) times the gradient at that point, and
- Repeat until convergence.
As I understand, this is a rather technical description. Let’s think about a bowl of cereals. The bowl is the plot of the cost function and its bottom would be the minimum of the cost function. A random position on the surface of the bowl is the current value of the coefficients. The goal is to compute the value of the cost function for different values for the coefficients and select the coefficients that give a smaller cost. Then, we would repeat this process until we get the coefficients that give the minimum cost, i.e. the bottom of the bowl.
As part of my PhD, I am using the gradient descent algorithm to do parameter estimation in a fluid-structure-interaction model that is often used for establishing haemodynamics models.
23/02/2022 - Haibo (ESR #3)

This flyer shows the promise of computer-assisted predictive analytics in cardiovascular safety evaluation. Predictive analytics models are designed to discover patterns in historical data, observe trends and use that information to make a prediction on new data. In drug development and safety evaluation studies, researchers usually need to analysis a large amount of data (“big data”) generated by these experiments. For example, the field potentials (FP) recordings of hiPSC-CMs obtained by multi-electrodes arrays (MEA) technology can offer researchers hours of recordings (each data point can be one millisecond) from a large number of electrodes. So, handling this “big data” and make analysis become challenging. Predictive analytics models help the researchers to improve the efficiency and set up drug high-throughput screening procedures.
As part of my PhD project, I will investigate the usage of classification models like statistical and machine learning based models for drug safety testing. We have started using the classification model to answer questions like: can we reliably infer the impact of a new drug compound on the potassium channels of cardiomyocytes? After proper training and validation, the model automatically detects patterns of the normal state and changes induced by drug in electrical signals. A well-trained classification model and associated visualisation dashboard could significantly improve the speed of drug toxicity study.
References:
- F. Raphel, T. De Korte, D. Lombardi, S. Braam, J.F. Gerbeau, “A greedy classifier optimization strategy to assess ion channel blocking activity and pro-arrhythmia in hiPSC-cardiomyocytes,” PLoS Computational Biology, vol. 16, no. 9, pp. e1008203, Sep, 2020. DOI: 10.1371/jour- nal.pcbi.1008203
16/02/2022 - Elham (ESR #8)

Keeping laboratory animals in group housing is important for the animal welfare and it is mandated by law in many countries. Rodents are one of the social animals mostly used in pharmacology studies. Telemetry implants with tracking functionality could help to track the rodents in group housing experiment designs. In safety pharmacology studies, cardiovascular data of laboratory animals is typically acquired by wireless implantable telemetry devices. This technique enables the animals to freely move without external disturbances during experiments. Adding animal tracking to the telemetry system, enables us to better monitor the animals during cardiovascular safety pharmacological studies.
Video can help to track non-rodents and therefore support the analysis of the cardiovascular data. Any significant fluctuation in the ECG signals and Cardiovascular data, can be correlated to movement artefacts by video analysis. However, video tracking in rodents in a group house arena is more challenging. They are small and quickly in movement and changing posture.
In my PhD project, I will work on tracking micro chip-set to assist video tracking during cardiovascular telemetry data capturing in our laboratories. I will investigate the variability of cardiovascular and behavioural data to improve animal welfare in the context of in vivo safety pharmacological evaluations which will be used to analyse and quantify the outputs.
References:
- Prior, H., & Holbrook, M. (2021). Strategies to encourage the adoption of social housing during cardiovascular telemetry recordings in non-rodents. Journal of Pharmacological and Toxicological Methods, 108, 106959. https://doi.org/10.1016/j.vascn.2021.106959
- Weber, E. M., Dallaire, J. A., Gaskill, B. N., Pritchett-Corning, K. R., & Garner, J. P. (2017). Aggression in group-housed laboratory mice: why can’t we solve the problem? Lab Animal, 46(4), 157–161. https://doi.org/10.1038/laban.1219
- Markert, M., Trautmann, T., Krause, F., Cioaga, M., Mouriot, S., Wetzel, M., & Guth, B. D. (2018). A new telemetry-based system for assessing cardiovascular function in group-housed large animals. Taking the 3Rs to a new level with the evaluation of remote measurement via cloud data transmission. Journal of Pharmacological and Toxicological Methods, 93, 90–97. https://doi.org/10.1016/j.vascn.2018.03.006
- Home. (n.d.). Retrieved February 03, 2021, from https://www.tse-systems.com/
- Peleh, T., Bai, X., Kas, M. J., & Hengerer, B. (2019). RFID-supported video tracking for automated analysis of social behaviour in groups of mice. Journal of Neuroscience Methods, 325, 108323. https://doi.org/10.1016/j.jneumeth.2019.108323
09/02/22 - Dustin (ESR #14)

In my PhD-project, I investigate the cardiotoxic effects of doxorubicin, a common chemotherapeutic drug. This drug is one of the most used and highly effective chemotherapeutics. I specifically look for the earliest signs of these cardiac adverse effects. Thus, patients at risk can be identified before they even develop severe cardiac injuries. If you want to find out how I do this check out my previous flyer!
My PhD project is conducted at the University of Antwerp, where we have the possibility to run in vivo, in vitro or ex vivo experiments. However, in my experience our research is limited to the expertise and infrastructure available in our labs. Within the INSPIRE project, I have the chance to increase my scientific outcome by combining expertise and facilities from both universities and companies.
In the beginning of 2022, I will spend a few weeks at UCB Pharma in Brussels to perform additional experiments at UCB’s state-of-the-art facilities. More specifically, together with my UCB colleague Martina Cherubin 'ESR #2), I will perform highly specific molecular analysis of possible biomarkers (microRNAs) in the blood and heart samples, previously collected at the University of Antwerp. Eventually, this will lead to a better understanding of the (molecular) damage this specific chemotherapeutic can cause.
02/02/22 - Tommaso (ESR #6)

What you see in the picture is one of the setups we use in the lab to observe behavior exhibited by the mice, which are very similar to those we can find in psychiatric disorders. The four arenas you see [1] can be used to simulate many different conditions (anxiety, stress disorders, autism...etc.) and so this system can be suited to the study of different psychopathologies. In my particular case, I am interested in reproducing a PTSD-like condition and correlating it with possible cardiovascular alterations.
Why are the mice colored? Well, we actually paint the fur of these mice with different colors to both help us to distinguish between the mice more easily and to help the DeepLabCut algorithm too. Setups like this can greatly improve behavioral studies, as previously explained, we leave the mice in a semi-natural environment with the best conditions for them to exhibit the behavior we are interested in, instead of forcing the animals. The advantages of this approach are a better quality of our results and an improved quality of life for the animals overall.
If you are interested and want to know more check out the reference.
Reference:
- Shemesh et al. 2013, High-order social interactions in groups of mice, eLIFE, http://dx.doi.org/10.7554/eLife.00759.001
26/01/22 - Patrizia (ESR #12)
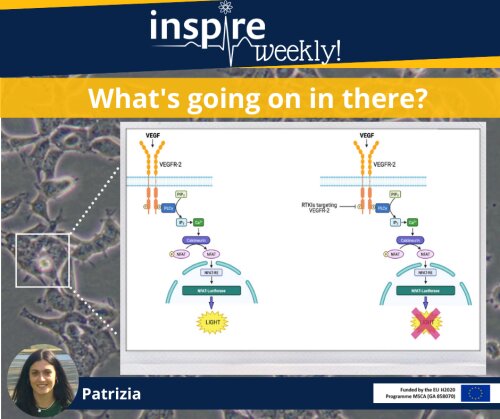
Vascular endothelial growth factor (VEGF) is an important signalling molecule and an established target for anticancer therapies. As a result, the development and approval of RTKIs (receptor tyrosine kinase inhibitors) targeting VEGFR-2 have provided an important therapeutic strategy in the oncology field. However, an impaired cardiovascular function has been associated with these novel targeted therapies. Among the cardiovascular dysfunctions associated with these antiangiogenic drugs, hypertension represents the most common adverse effect. From the clinical evaluation of RTKI targeting VEGR-2, the incidence of hypertension appears to be associated with the potency of these therapeutics against VEGFR-2, such that agents with higher potency are associated with a higher incidence of hypertension 1. During the first part of my PhD, I quantitatively measured the potency of these drugs, using the NFAT reporter gene assay. The binding between VEGFR-2 and its ligands initiates a signalling cascade, leading to activation of calcineurin and consequent dephosphorylation of the transcription factor NFAT. Next, NFAT translocates to the nucleus where it promotes the transcription of pro-angiogenic and pro-inflammatory genes 2. By using HEK293T cells transfected with a luciferase gene, the NFAT reporter gene assay permits the quantification of the inhibitory effects of RTKIs on NFAT transcriptional activity 3. Indeed, RTKIs targeting VEGFR-2, by blocking VEGFR-2 signalling, determine a reduction of the NFAT-luciferase activity, thus producing a decrease of luminescence. The resulting pIC50 values provided information about their potency, which will be essential for the subsequent in vivo characterisation of the cardiovascular consequences of these drugs.
References:
- León-Mateos, L., Mosquera, J. & Antón Aparicio, L. Treatment of sunitinib-induced hypertension in solid tumor by nitric oxide donors. Redox Biol 6, 421-425 (2015).
- Simons, M., Gordon, E. & Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol 17, 611-625 (2016).
- Hill, S.J., Baker, J.G. & Rees, S. Reporter-gene systems for the study of G-protein-coupled receptors. Curr Opin Pharmacol 1, 526-532 (2001).
19/01/2022 - Tommaso (ESR #6)

Today, we talk a little bit about tracking systems! Tracking systems are incredibly useful and powerful when it comes to analyzing animal behavior based on observations.
Just try to imagine yourself busy watching hours and hours and hours of videos with animals freely moving in a determined space and having to try to understand the behavior exhibited. Not only it would require a huge effort, but it would also be very prone to mistakes if the person in charge has to do everything by him/herself.
There are different approaches to achieve good tracking. Generally speaking, we can say that they all require a combination of hardware and software.
The images you are obtained with one of the most popular system of tracking called DeepLabCut [1][2]. DeepLabCut is a a software that uses neural network processes to train algorithms and uses these to track the animals in the videos. A great advantage of this system is that require only a camera, the DeepLabCut software (which is open-source), and a little will to learn how it works. Check out the references to learn how can you use it!
References:
- Mathis et al. 2018, DeepLabCut: markerless pose estimation of user-defined body parts with deep learning, Nature Neuroscience, https://doi.org/10.1038/s41593-018-0209-y
- Nath et al. 2019, Using DeepLabCut for 3D markerless pose estimation across species and behaviors, Nature protocols, https://doi.org/10.1038/s41596-019-0176-0
12/01/2022 - Marieke (ESR #11)

Photinus pyralis, commonly called fireflies or lightning bugs, use bioluminescence to attract mates or prey. To do this, they produce an enzyme, a luciferase, which catalyses a reaction resulting in the production of light. In pharmacological studies, this enzyme can be used to study the interaction of a drug with its target or with other components of the cells.1 Similarly, luciferases from Oplophorus gracilirostris, a deep sea shrimp emitting light as a defence mechanism, or Renilla luciferases, derived from a sea pansy, can be used to study the interaction between drugs and their targets.1 In my project, I use luciferases to study how anticancer drugs affect receptors that are involved in the development of cardiovascular side effects. In isolated human kidney cells where a luciferase is attached to the receptor, we can measure the amount of light that is produced by these cells after drug treatment.2,3 In this way, we determine how strongly or weakly the drug is binding to the receptor and we gather information on the side effects patients might have when taking these drugs.1–3
References:
- Hall MP, Unch J, Binkowski BF, et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem Biol 2012; 7: 1848–1857.
- Carter JJ, Wheal AJ, Hill SJ, et al. Effects of receptor tyrosine kinase inhibitors on VEGF165a- and VEGF165b-stimulated gene transcription in HEK-293 cells expressing human VEGFR2. Br J Pharmacol 2015; 172: 3141–3150.
- Kilpatrick LE, Alcobia DC, White CW, et al. Complex Formation between VEGFR2 and the β2-Adrenoceptor. Cell Chem Biol 2019; 26: 830-841.e9.
15/12/2021 - Callan (ESR #10)
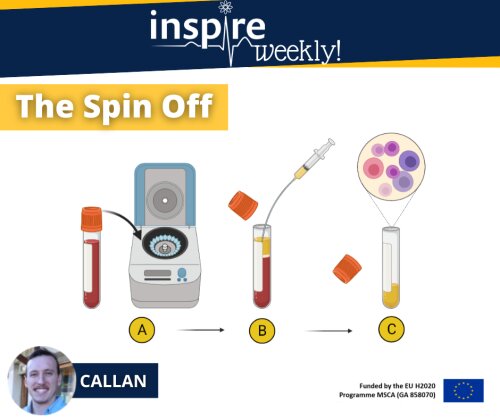
In Safety Pharmacology, an important element of drug research and development, it is common practice to determine the blood concentration of a drug candidate over time. This is important information to understand the drug exposure-response relationship (i.e., PharmacoKienetic-Pharmacodynamic (PK-PD modelling)). Of note, only a free, unbound drug will interact with its molecular target. Furthermore, plasma concentrations can be used to investigate and calculate drug exposure of specific tissues. During drug development, PK-PD modelling will guide the researchers to determine the appropriate therapeutical dose, while avoiding adverse events due to exposure to toxic doses.
In this INSPIRE Weekly post, I explain the procedure to obtain plasma from collected blood samples:
The first step (A) involves placing the blood sample tube into a centrifuge. This device uses centrifugal forces to separate the various components within your blood whilst spinning them at high speed around its axis (Stephenson. 2010). The heavier and denser red blood cells will sink to the bottom of the tube whilst the lighter, less dense plasma will rise to the top of the tube. Leaving a clear separation between your red blood cells and your plasma as seen in step B.
The collection step (B) is done by making use of a pipette or syringe and drawing all the lighter less dense plasma (as depicted in yellow) from the tube.
The collected plasma (c) is then stored in a separate tube at -80°C until molecular analysis is performed to predict the concentration of the drug present in the plasma of the subject.
References:
- Rizk, M. L., et al. (2017). "Importance of Drug Pharmacokinetics at the Site of Action." Clin Transl Sci 10(3): 133-142.
- Frank H. Stephenson. (2010). “Calculations for Molecular Biology and Biotechnology.” (Second Edition).
- Tillement, J.-P. (1986). “Protein binding and drug transport: Symposium.” Schattauer.
08/12/2021 - Sara (ESR #8)
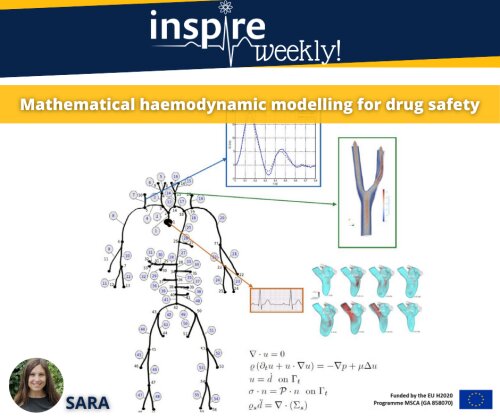
Mathematical modelling studies are increasingly recognised as an important tool used in drug development [1]. There is a great variety of ways in which mathematics can play a role in drug discovery and development. Sometimes, quantitative information about the underlying mechanism of action is limited, or cannot be measured, and mathematical modelling can help to understand the effect of drug exposure. A successful model combines physiological information, well-designed tests and numerical investigation and can be utilized to create reliable predictions.
The main goal of my PhD is to develop novel in silico models to describe the effect of drugs on the cardiovascular function. Several experimental data will be used in order to iterate and validate the model [2]. The major role of the model, besides providing a quantitative insight into the investigated phenomena, is to make it possible to relate measurements with predictions, which are usually parameters that cannot be directly measured. Currently, there are some mathematical models available, like fluid-structure-interaction (FSI) models, that couple the fluid (blood) and the arterial wall [3]. The goal of my PhD is to develop suitable models for the estimation of drug effects in the cardiovascular system from measured data.
References:
- Peletier, L. A., & Gabrielsson, J. (2018). Impact of mathematical pharmacology on practice and theory: four case studies. Journal of pharmacokinetics and pharmacodynamics, 45(1), 3–21. https://doi.org/10.1007/s10928-017-9539-8
- Guns, P. D., Guth, B. D., Braam, S., Kosmidis, G., Matsa, E., Delaunois, A., Gryshkova, V., Bernasconi, S., Knot, H. J., Shemesh, Y., Chen, A., Markert, M., Fernández, M. A., Lombardi, D., Grandmont, C., Cillero-Pastor, B., Heeren, R., Martinet, W., Woolard, J., Skinner, M., … Valentin, J. P. (2020). INSPIRE: A European training network to foster research and training in cardiovascular safety pharmacology. Journal of pharmacological and toxicological methods, 105, 106889. https://doi.org/10.1016/j.vascn.2020.106889
- Formaggia, Luca & Quarteroni, Alfio & Veneziani, Alessandro. (2009). Cardiovascular Mathematics: Modeling and Simulation of the Circulatory System. 10.1007/978-88-470-1152-6.
01/12/2021 - Elham (ESR #7)

Wireless monitoring with implantable telemetry devices enables animals to freely move without external disturbances during safety pharmacology experiments. This technique increases animal welfare by allowing a continuous recording of their cardiovascular parameters with just one implantable microchip and a receiver in close proximity to the housing unit. But, what about if we could give the animals more space in that they could even exceed the range of the receiver?
Telemetry-based implants with internal memory offer us to remotely record cardiovascular data away from the receiver without losing data. During cardiovascular experiments, laboratory animals can move around freely with flexibility in the location, and all the data will be continuously collected in the implanted memory chip. Data will be transmitted whenever the implant is within range of the receiver. This free movement increases the life quality of laboratory animals by playing around and interact together, which contributes to 3Rs (replacement, refinement, and reduction) in in-vivo experiments. Consequently, it has a positive effect on reliability and accuracy of cardiovascular data in safety pharmacology studies.
During my Ph.D. project, I will investigate this telemetry system, with the ability to store real-time cardiovascular data in its memory. The gathered cardiovascular data will qualify, cluster, and validate for each experiment with high quality data and increased level of “freedom to operate” of animals.
References:
- Markert, M., Trautmann, T., Krause, F., Cioaga, M., Mouriot, S., Wetzel, M., & Guth, B. D. (2018). A new telemetry-based system for assessing cardiovascular function in group-housed large animals. Taking the 3Rs to a new level with the evaluation of remote measurement via cloud data transmission. Journal of Pharmacological and Toxicological Methods, 93, 90–97. https://doi.org/10.1016/j.vascn.2018.03.006
- Home. (n.d.). Retrieved February 03, 2021, from https://www.tse-systems.com/
24/11/2021 - Patrizia (ESR #12)

During the last decade, cancer treatment has undergone a revolutionary progress, moving from non-selective cytotoxic therapies to novel molecular targeted drugs, specifically addressed to cellular pathways overexpressed in cancer cells but not in normal cells 1. Many of these new antineoplastic therapeutics (i.e., Vascular Endothelial Growth Factor (VEGF) inhibitors) have significantly improved cancer prognosis and cancer survivorship. However, several cardiovascular complications have emerged as associated to these therapies (i.e., VEGF inhibitors often cause hypertension, left ventricular dysfunction and thromboembolism), without being anticipated in preclinical studies 2. As a result of these cardiovascular adverse sequelae related to novel anticancer target therapies, the new discipline of cardio-oncology has emerged 3. This multidisciplinary field is focused on limiting and predicting cardiovascular dysfunctions in cancer patients and survivors 4, with the dual aim of reducing mortality for cardiovascular complications in cancer patients, as well as improving our understanding of signalling events implicated in these manifestations.
How do VEGF inhibitors induce cardiovascular toxicities? Several hypotheses have been proposed to explain cardiovascular complications associated with anti VEGF therapies, which either prevent activation of VEGF-receptors (i.e., VEGF antibodies) or interfere with signal transduction (i.e., receptor tyrosine kinase inhibitors (RTKIs)). However, a clear understanding of the processes by which VEGF inhibition impairs cardiovascular function remains to be elucidated 5. During my PhD I will investigate the possible mechanisms underpinning RTKI-induced cardiovascular toxicities. Using preclinical models for the haemodynamic assessment of anti-VEGF therapies represents a valuable method to predict haemodynamic changes associated with these drugs, in addition to permitting the understanding of the mechanisms responsible for RTKIs-related cardiovascular toxicity.
References:
- Gerber, D.E. Targeted therapies: a new generation of cancer treatments. Am Fam Physician 77, 311-319 (2008).
- Sheng, C.C., et al. 21st Century Cardio-Oncology: Identifying Cardiac Safety Signals in the Era of Personalized Medicine. JACC: Basic to Translational Science 1, 386-398 (2016).
- Campia, U., et al. Cardio-Oncology: Vascular and Metabolic Perspectives: A Scientific Statement From the American Heart Association. Circulation 139, e579-e602 (2019).
- Guha, A., Armanious, M. & Fradley, M.G. Update on cardio-oncology: Novel cancer therapeutics and associated cardiotoxicities. Trends Cardiovasc Med 29, 29-39 (2019).
- Small, H.Y., Montezano, A.C., Rios, F.J., Savoia, C. & Touyz, R.M. Hypertension due to antiangiogenic cancer therapy with vascular endothelial growth factor inhibitors: understanding and managing a new syndrome. Can J Cardiol 30, 534-543 (2014).
17/11/2021 - Tommaso (ESR #6)

Today we are talking about environmental enrichment! This is one of those measures that can be taken in order to increase the quality of life of our lab animals (look at flyer from 13/10/2021 - below), but that's not the only purpose. In fact, environmental enrichment increases the complexity of the system used in the experiment and very often can represent a more ethological condition, which has two consequences:
- general increased quality of the data collected since the animals are in a more natural environment
- the possibility to simulate or observe more easily complex behaviors, which are the starting point for the study on pathologies like schizophrenia, autism, PTSD, and many other psychiatric disorders
What you see in the images are just some examples of the objects we use to enrich the environment of our mice
Shelter: it gives mice a nice and closed space (just how they like it!) to explore and rest and occasionally to hide from a confrontation with other males
Closed nest: it is a place where mice can sleep altogether to provide warmth to each other
Ramp: mice can climb to the top or jump from it. It also provides some variety to the environment and satisfies their explorative instinct
10/11/2021 - Haibo (ESR #3)
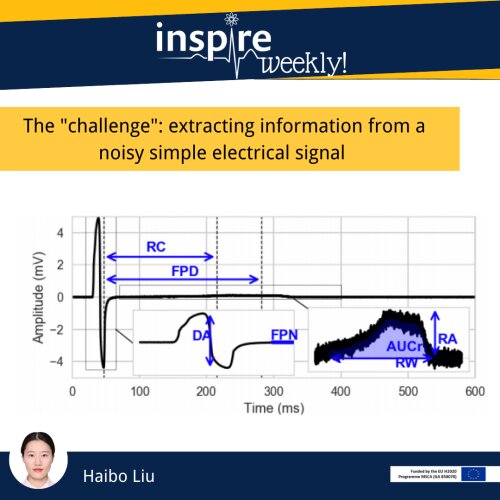
This flyer shows feature extraction from a type of field potential (FP) of human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs) obtained by multi-electrode array (MEA) technology [1]. The challenge dealing with FP data is the high dimension curse which causes problems related to costs of data processing and computation. The reason is that any simple operation on the high dimensional FP will cost tremendous computing resources compared to low dimensional data. Today, we would like to present a few matters about signal processing to overcome the high dimension curse.
So, we introduce the feature extraction technique which is a common signal processing technique that can reduce the dimension of the raw signal data and extract the important information in the FP. One example in the flyer is to compute Depolarization Amplitude (DA), DA is computed by using the maximum amplitude minus the minimum amplitude. By defining similar methods as DA computation to compute meaningful features, we could observe changes in the FP signals induced by drug toxicity through specific features instead of dealing with high dimensional signals directly. Because of the manageable data size of the extracted features, we could also speed up the training phase and reduce the computation costs of further modelling.
Reference:
- F. Raphel, T. De Korte, D. Lombardi, S. Braam, J.F. Gerbeau, “A greedy classifier optimization strategy to assess ion channel blocking activity and pro-arrhythmia in hiPSC-cardiomyocytes,” PLoS Computational Biology, vol. 16, no. 9, pp. e1008203, Sep, 2020. DOI: 10.1371/jour- nal.pcbi.1008203
03/11/2021 - Christian (ESR #4)

Telemetry technology gives researchers a high degree of flexibility and freedom to combine cardio-physiological experiments with a wide range of other experiments.
During my PhD project I will collaborate with the Wageningen University to combine telemetry with metabolic and nutritional studies. There, we will perform Indirect Calorimetry studies with the PhenoMaster (TSE Systems) platform for mice to measure whole body energy expenditure and substrate usage measured as respiratory exchange ratio1. Integrated microbiome gas sensors enable us to additionally measure (in exhaled air) the gut microbial fermentation products methane and hydrogen from indigestible foods2. This gives insights on how different nutritional treatments directly affect intestinal bacteria composition and their activity3, which influence not only gut health itself, but also brain function4.
Such metabolic studies are thus far performed in individually housed animals to quantify individual food intake, energy expenditure and other metabolic and physiological parameters. In line with the Directive 2010/63/EU we aim to house mice in pairs for animal welfare reasons. The novel Stellar telemetry implant (TSE Systems) with an integrated tracking functionality will track the mice individually and help to assign food and drink intake to each individual mouse. Furthermore, we aim to allocate the indirect calorimetry air measurements to the individual level as well.
References
- Hoevenaars, F. P., Keijer, J., Swarts, H. J., Snaas‐Alders, S., Bekkenkamp‐Grovenstein, M., & van Schothorst, E. M. (2013). Effects of dietary history on energy metabolism and physiological parameters in C57BL/6J mice. Experimental physiology, 98(5), 1053-1062. https://doi.org/10.1113/expphysiol.2012.069518
- Fernández-Calleja, J. M., Konstanti, P., Swarts, H. J., Bouwman, L. M., Garcia-Campayo, V., Billecke, N., ... & van Schothorst, E. M. (2018). Non-invasive continuous real-time in vivo analysis of microbial hydrogen production shows adaptation to fermentable carbohydrates in mice. Scientific reports, 8(1), 1-16. https://doi.org/10.1038/s41598-018-33619-0
- Breath reveals how gut bacteria process food, WUR impact story. https://www.wur.nl/en/article/breath-reveals-how-gut-bacteria-process-food.htm
- Cryan, J. F., O'Riordan, K. J., Cowan, C. S., Sandhu, K. V., Bastiaanssen, T. F., Boehme, M., ... & Dinan, T. G. (2019). The microbiota-gut-brain axis. Physiological reviews. https://doi.org/10.1152/physrev.00018.2018
27/10/2021 - Brigitta (ESR #1)
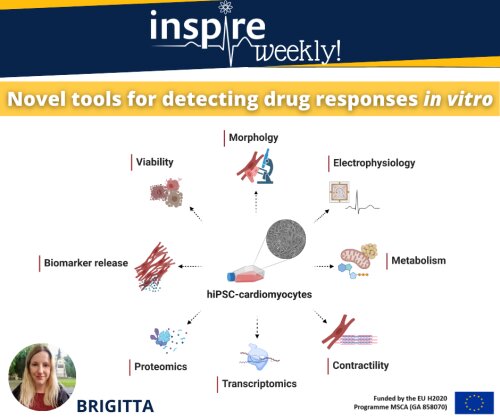
Cardiotoxicity after acute or chronic exposure to compounds is still one of the most common adverse drug side-effects. Changes in electrophysiological properties of cardiomyocytes, leading to arrhythmia are amongst the most common mechanisms. Predictive models to precisely determine the risk of these events with high specificity and sensitivity are lacking. Human induced pluripotent stem derivatives such as cardiomyocytes (hiPSC-CMs) present novel alternatives for drug safety assessment.
For detecting electrophysiological changes in hiPSC-CMs patch-clamp techniques – allowing high precision but low-throughput – can be utilized. More scalable options include multi electrode array (MEA) recordings and optical mapping of voltage sensitive dyes. Disruption in the ATP-generating pathways and the state of mitochondrial network also significantly impact on cardiac health. Thus, monitoring drug-induced dysfunction in these processes via measuring oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) as well as dyes targeting mitochondrial membrane potential and oxidative state contribute to evaluating cardiac safety. Moreover, a number of functional assays have been established for measuring changes in contractility and calcium handling in single-cells, 2D monolayers and 3D constructs of hiPSC-CM (co)cultures.
Big data science complemented by new generation analytic techniques for transcriptomics, proteomics and biomarker identification can be coupled to phenotypic assays to reveal underlying mechanisms of toxicity or be used as predictive tools. Studying viability and more detailed morphological features, utilizing high content imaging methods for dyes selectively staining specific organelles or cellular membranes can shed light on structural toxicity. Readouts from the above-mentioned assays individually or as a combination can serve as building blocks of in vitro cardiac safety scores with translational value to the clinic.
As part of my PhD project, we will focus on further validating and standardizing protocols for several of these assays.
20/10/2021 - Dustin (ESR #14)
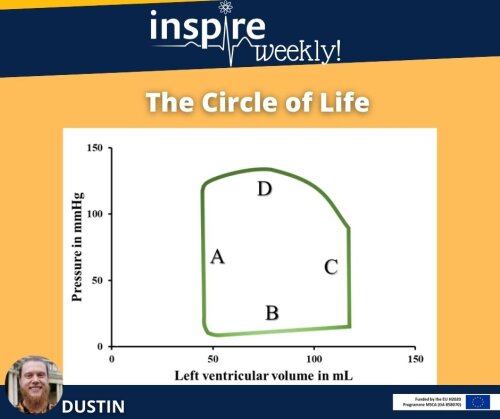
In today’s INSPIRE weekly post, I present the “circle of life”. This graph shows the volume and pressure relationship during a single heart beat in the left ventricle presented as a loop. A heart beat can be described in 4 phases displayed in the image (A-D). The cardiac cycle starts with the so-called isovolumetric relaxation of the heart (A). This means that the heart muscle cells relax while the volume of blood in the chambers is constant. The second phase (B) is the ventricular filling where blood enters the heart chamber and the pressure in the heart increases. After a certain threshold is reached the heart contracts and builds up pressure (C), which leads to ejection of the blood (D) from the heart into the aorta. After the ejection of the blood, the cardiac cycle starts again with phase A.
These phases can be simplified as diastole (phase A and B) and systole (phase C and D). Diastole describes the relaxation and filling of the heart. Whereas systole the ejection and contraction includes.
In my project I will determine pressure volume loops via in invasive catheter in mice treated with known cancer drugs to precisely investigate changes in systole and diastole.
13/10/2021 - Tommaso (ESR #6)

The use of animals in experiments is something very important to all of us, scientists or not. In the past decades, a lot of progress has been made to replace lab animals where possible and a lot of work is still ongoing nowadays. In general, scientists operate following the rules known as the "3Rs", which are hierarchically organized:
- Replacement = Use a model that is not an animal if possible
- Reduction = Use the least amount of animal labs that still allows getting significant data
- Refinement = Give the animals the best conditions possible
In the image here you find a gross representation of the proportion of the animals used in scientific research and here there is a table with the exact percentages [1].
- Mice: 2,92%
- Fish: 14,61%
- Rats: 5,05%
- Birds: 4,21%
- Other mammals: 2,36%
- Reptiles : 0,003%
- Amphibians: 0,28%
- Primates: 0,09%
- Cats: 0,005%
- Dogs: 0,13%
- Horses: 0,30%
References:
- Robinson et al. (2019), The current state of animal models in research: A review, International Journal of surgery, https://doi.org/10.1016/j.ijsu.2019.10.015
21/07/2021 - Benji (ESR #13)
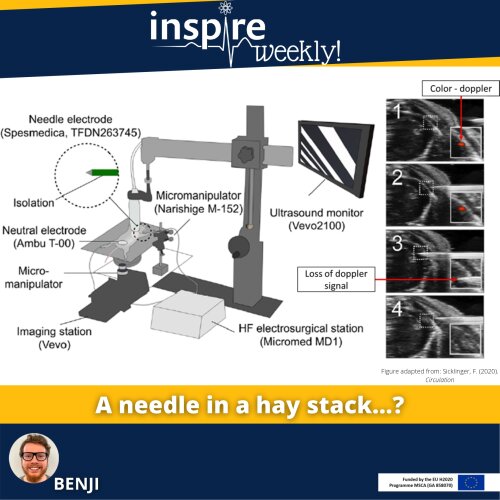
Myocardial infarction (MI) is a common cause of heart failure (HF). Therefore, induction of an MI is often used to study the resulting HF in mice. To induce MI in mice, several surgical methods have been developed over the past decades. Surgical ligation of the left anterior descending coronary artery (LAD) is the most commonly used method. Sicklinger et al. recently published a new method, which employs a cardiac ultrasound for LAD localization and a coagulator to induce a MI.
The method of Sicklinkger et al. uses a high frequency (HF) electrical impulse to coagulate the LAD. To this end, a micromanipulator-controlled needle is inserted into the chest and navigated to the LAD under ultrasound guidance. This new technique allows real-time assessment of cardiac function, infarct size and improves stratification post-procedure. In addition, the duration of the procedure is reduced from 20–30 minutes to 6–8 minutes and, importantly, the procedure is minimally invasive and improves recovery of the mice.
During my PhD, we will set-up and use this method in our lab to induce HF in a colon cancer mice model previously shown to have enhanced cancer growth in the presence of HF. Next, we will study the role of endothelial-derived growth factors as a potential integrator of the pathophysiology of HF and cancer.
14/07/2021 - Anna (ESR #15)
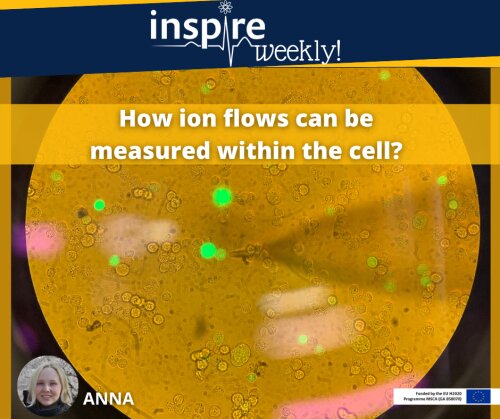
Electrophysiology is the branch of physiology investigating into the flow of ions (ion currents) using electrical recording techniques that enable the measurement of these currents. In my laboratory practice, I am using a common tool of electrophysiologists, a patch clamping experiment, which is an assay to evaluate ion flows of the cell. We are using this assay as the heart cells (cardiomyocytes) for beating require ion currents to interexchange between inner and outer parts of the cellular membrane, where the most important currents are potassium (K+), sodium (Na+) and calcium (Ca2+) [1]. A number of transporters for each of the ion currents are intercalated into the membrane serving as a gate opening upon change of the membrane’s electrical charge.
Therefore, to study the gating ability of ion channels in cells exposed to a drug, we apply a voltage range and measure the occurring response as an ion flow. For the electrical impulse and subsequent recording, two electrodes are used. One is in the microscope’s bath with the cells and another is situated in the glass micropipette, with a tip diameter of < 1 micrometre, which you can see approaching the cell on the photo. Here, I am going to record an ion flow in Chinese hamster ovary (CHO) cells in response to a drug causing side effect on human cardiomyocytes. Thereby, during my PhD project (‘’Personalized safety pharmacology against drug-evoked proarrhythmia’’), I would like to investigate the mechanisms when this off-target effect occurs.
References:
- Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol. 2009 Apr;2(2):185-94.
07/07/2021 - Martina (ESR #2)

To monitor the spread of the SARS-CoV-2 (COVID-19) and the number of infections, Health and Sanitary organizations have adopted several measures to counteract the pandemic, including testing patients for their positivity to the virus with qPCR tests. The quantitative Polymerase Chain Reaction (qPCR) is a laboratory technique that allows to detect genetic material from a specific organism, such as DNA- or RNA- viruses. But how it really works? Firstly, RNA transcripts are converted by reverse transcription into their complementary DNAs (cDNA); then, DNA molecules are amplified by 3 repeating steps: denaturation of the double strand DNA into single strands thanks to heat; annealing with the DNA’s primers; elongation, in which DNA polymerase extends the 3′ end of each primer along the template strands, until the complete formation of a new dsDNA. In this way, at every cycle, it is possible to exponentially increase the number of the targeted DNAs.
During my PhD, I am going to use the qPCR technique to investigate new biomarkers of cardiotoxicity - as, for example, the expression of miRNAs - on in vitro cultures of human induced Pluripotent Stem Cells - derived Cardiomyocytes (hiPSC-CMs), when exposed to some cardiotoxic drugs. Using qPCR, we will amplify selected miRNAs from hiPSC-CM and compare their expression to the control.
30/06/2021 - Brigitta (ESR #1)
Just over 15 years ago, the first induced pluripotent stem cells (iPSCs) were generated using mouse fibroblasts 1. Shortly after, induction of pluripotency in human fibroblasts was also proven possible 2, opening a whole new chapter of human based research paradigms.
Concurrent refinement of differentiation protocols paved the way for cell types such as iPSC-derived cardiomyocytes (iPSC-CMs) to routinely serve as disease models, platforms for drug testing, and enter clinical trials for cell therapies 3-5.
However, iPSC-CMs still resemble an embryonic phenotype compared to adult cardiomyocytes, leaving maturation as one of the most pressing challenges in the field. Approaches aiming to mimic biochemical and -mechanical cues that drive heart development in vivo have shown success in approximating the adult cardiac cellular phenotype. Methods such as applying electromechanical stimuli, modifying extracellular matrix, introducing nanotopology, 3D culture and adjusting medium composition also lead to a certain extent of structural and functional maturity, however, several limitations persist 6, 7.
As part of my project, I will be working on testing various platforms promoting iPSC-CM maturation with compatibility to high throughput screening.
References:
- Takahashi, K. and S. Yamanaka, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 2006. 126(4): p. 663-76.
- Takahashi, K., et al., Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 2007. 131(5): p. 861-72.
- Lian, X., et al., Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A, 2012. 109(27): p. E1848-57.
- Burridge, P.W., et al., Chemically defined generation of human cardiomyocytes. Nat Methods, 2014. 11(8): p. 855-60.
- Elliott, D.A., et al., NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat Methods, 2011. 8(12): p. 1037-40.
- Ahmed, R.E., et al., A Brief Review of Current Maturation Methods for Human Induced Pluripotent Stem Cells-Derived Cardiomyocytes. Front Cell Dev Biol, 2020. 8: p. 178.
- Karbassi, E., et al., Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nature reviews. Cardiology, 2020. 17(6): p. 341-359.
23/06/2021 - Christian (ESR #4)

The use of telemetry technology to measure cardiovascular parameters is a core principle during safety pharmacology studies. Most of these studies are performed with laboratory animals kept in single cages, which is a well-known stress factor. It has also been shown that group-housing has some beneficial effects on the cardiovascular system1,2. Therefore, safety pharmacology strives to advance future studies from single to group house environments to stimulate safer drug development.
A novel Stellar Telemetry implant with integrated micro-GPS functionality, of which its development and validation is the main objective of my PhD project, offers a new technology for researcher to analyse cardiovascular parameters and behavioural traits simultaneously in socially interacting animals, which has been neglected in safety pharmacology so far. This technology ensures the possibility to keep laboratory animals in groups, which reflects their natural habitat and thereby minimizes stress.
Beyond the development of innovative technologies, TSE Systems designs group-housed environments in which such experiments can be performed and combined with automated behavioural phenotyping. The PhenoWorld (picture) is a new concept which raises animal housing and experimentation onto the next level by combining different measurement compartments with highly enriched living environments3.
Future laboratory animal experiments performed in such multilayer quarters will contribute to elucidate possible improvements on animal welfare aspects and on data quality, which could – for example – lead to the development of new and safer drugs.
References:
- Xing, G., Lu, J., Hu, M., Wang, S., Zhao, L., Zheng, W., ... & Skinner, M. (2015). Effects of group housing on ECG assessment in conscious cynomolgus monkeys. Journal of pharmacological and toxicological methods, 75, 44-51. https://doi.org/10.1016/j.vascn.2015.05.004
- Späni, D., Arras, M., König, B., & Rülicke, T. (2003). Higher heart rate of laboratory mice housed individually vs in pairs. Laboratory animals, 37(1), 54-62. https://doi.org/10.1258/002367703762226692
- Castelhano-Carlos, M. J., Baumans, V., & Sousa, N. (2017). PhenoWorld: addressing animal welfare in a new paradigm to house and assess rat behaviour. Laboratory animals, 51(1), 36-43. https://doi.org/10.1177/0023677216638642
16/06/2021 - Dustin (ESR #14)
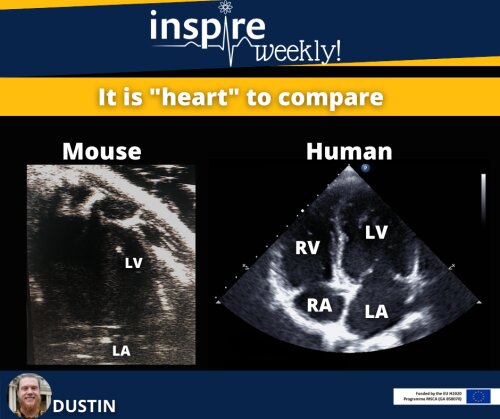
This flyer shows an image acquired by echocardiography. It is a comparison of ultrasound images of a heart of mouse (left) and a human (right). *Disclaimer: The images are taken with a different resolution and have a different scale. The heart consists of 4 chambers divided in two small- (atria) and two large chambers (ventricles). The blood enters the heart via the atrium. Next, the blood is collected in the ventricle (diastole) and pumped out with every heart beat (systole).
These types of images are routinely used to examine the heart of patients, since it presents safe, convenient and non-invasive way to investigate both anatomy (B-mode) and function (M-mode) of the heart. In a comparison the four-chamber-view of the two hearts is shown. This view is used to specific investigate the pumping behavior including the blood flow of all chambers and gives therefore insights about the diastolic and systolic function.
As seen in the image, the four-chamber view of the mouse does not always show all 4 chambers. In the mouse heart there is often just the left ventricle and atrium visible. This is caused by a shadow from the breast bone covering the right side of the mouse heart. However, the parameters measured by this method good concordance between mice and human. Consequently, I will use, among other techniques, high-resolution ultrasound imaging method in my project to examine the function of the left ventricle in mice during cancer therapy.
09/06/2021 - Anna (ESR #15)

What do you think scientists observe on a daily basis? The picture above reproduces the view from a microscope loaded with the animal cells. These are the cells of ovarian epithelium derived from Chinese hamsters (CHO). The hamsters are easy to breed, yet once the cells were isolated in 1957 by Theodor Puck 1, there is no need anymore to keep the living hamsters for cell production. The isolated CHO cells, like any other mammalian cells, have an ability to grow and divide while being maintained in a liquid medium containing all the essential nutrients such as glucose as carbohydrate, amino acids for protein production and vitamins for the activity of enzymes within the cell. As the cells are commonly arranged into tissues, such as epithelial ovarian tissue in case of CHO cells, they retain a tendency to arrange into groups and attach to a substrate. So that these CHO cells simply grow on the bottom of a plastic Petri dish filled up with the nutrient medium. The simplicity of maintenance, cellular line stability and similarity of produced proteins to human, made CHO cells a well-known system for the production of recombinant proteins for industrial and research purposes 2. Recombinant proteins are produced by the methods of genetic engineering and incorporate the genetic material from different sources. For instance, on the picture, CHO cells were cloned (i.e. transfected) with a gene originated from jellyfish producing green fluorescent protein (GFP) 3, the glow of which is not visible under the light microscope, but you can observe its fluorescence in the next post. Another transfected gene was the gene of an ion channel. Since CHO cells do not express any cardiac-related ion transporters 1, it is a convenient system to study an ion channel of interest without any influence of other channels present in human heart tissue.
Thus, as a part of my PhD project, I am using CHO cells as a model organism to study the pharmacological effects of drugs on the cellular level. I examine these cells using a patch-clamp technique, about which I will elaborate in the next post.
References:
- Gamper N, Stockand JD, Shapiro MS. The use of Chinese hamster ovary (CHO) cells in the study of ion channels. Journal of Pharmacological and Toxicological Methods. 2005 May; 51(3):177-85
- Kim JY, Kim YG, Lee GM. CHO cells in biotechnology for production of recombinant proteins: current state and further potential. Appl Microbiol Biotechnol. 2012 Feb;93(3):917-30
- Remington SJ. Green fluorescent protein: a perspective. Protein Sci. 2011 Sep;20(9):1509-19
02/06/2021 - Sara (ESR #8)
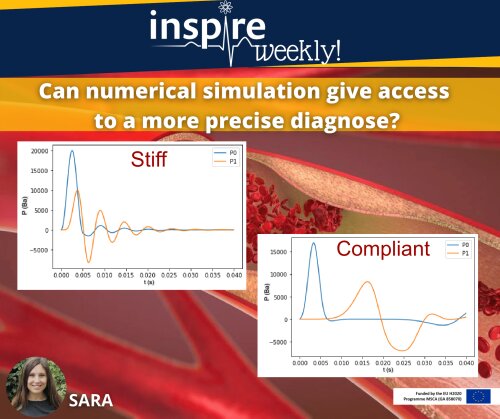
In the poster, you see pressure signals obtained from in silico modelling in different conditions, i.e. in a compliant (healthy) and stiff (aged) blood vessel. The picture shows P0 (blue), which is the pressure wave over time measured close to the beginning of a tube, while P1 (orange) is the pressure measured further down the vessel, assuming a cylindrical elastic tube set-up. The left picture corresponds to an aged or diseased blood vessel with a Young modulus of 2.5 ∙106 dyn·cm−2 . The right graph shows a healthy blood vessel with a Young modulus of 0.5 ∙106 dyn·cm−2. The Young modulus represents the stiffness of the vessel and has been correlated to arterial ageing and cardiovascular disease, such as arterial calcification.
Mathematical modelling and numerical simulation help to obtain insight in the relation between wall parameters such as the Young’s modulus and pulse wave behavior. Moreover, increased pulse wave velocity (PWV) as well as deviation in the morphology are risk factors for cardiovascular disease. As such, mathematical models provide a useful tool for safety pharmacologist to better understand the possible impact of drug-induced changes in hemodynamic parameters and how these translate into clinical risk depending on patient-specific characteristics.
26/05/2021 - Callan (ESR #10)
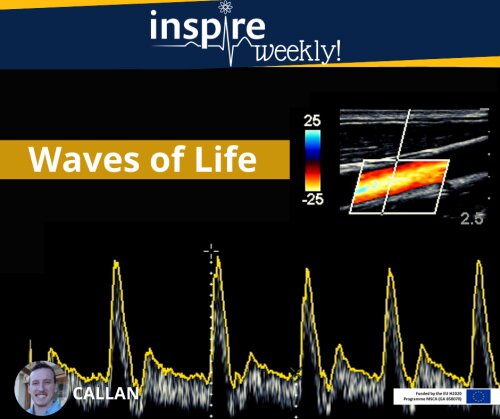
Ultrasound imaging is a common practice in clinical setups which has been around since the late 1950’s1. This technology is frequently used in pregnant women to gain insight into anatomical imaging2. However, our lab has implemented this technique for functional imaging of the cardiovascular system in rodents. The spatial resolution of conventional ultrasound imaging systems used in the clinic provides a barrier for pre-clinical imaging of rodents (rat or mice) given their small size. To overcome this challenge we will make use of transducers which have a much higher frequency to produce higher resolution images3. More specifically when investigating the aortic regions where the diameters (captured by B-Mode imaging) and the velocities of the blood flow (captured by Doppler imaging) will be recorded as presented in the images above. These images are then respectfully plotted side by side in order to calculate the pulse wave velocity which will provide us valuable insight into the local stiffness of the vasculature. The main goal and importance of my PhD project is for this technique to be used as a valuable tool in safety pharmacology testing for the screening of existing as well as potential new drug candidates.
References:
- Sprawls P. The Physical Principles of Medical Imaging, 2nd Ed. 1995, Medical Physics Pub. (Madison, Wis).
- Liff I, Bromley B. Fetal Anatomic Imaging Between 11 and 14 Weeks Gestation. Clin Obstet Gynecol. 2017 Sep;60(3):621-635. doi: 10.1097/GRF.0000000000000296. PMID: 28742595.
- Moran, C. M. and A. J. W. Thomson (2020). Preclinical Ultrasound Imaging—A Review of Techniques and Imaging Applications.Frontiers in Physics 8(124).
12/05/2021 - Marieke (ESR #11)

In this image, you can see a blood vessel segment mounted on two glass micro-cannulae. In this set-up, the environment of the vessel is maintained under physiologically relevant conditions. Acidity and temperature of the fluid around the vessel can be adjusted and the pressure both inside and outside of the vessel can be regulated. After the vessel is secured and the environment is prepared, this technique, called pressure myography, allows measurement of dynamic changes in the diameter of the vessel.1 Drugs can be tested, either in the bathing solution or intra-luminally; their vasoactive properties may cause a contraction of the vessel, so the diameter of the vessel segment will decrease, or cause a vasodilation, meaning the diameter will increase.
Vascular Endothelial Growth Factor (VEGF) inhibitors are powerful drugs to stop tumour growth, but they are known to cause an elevated blood pressure in patients taking them.2 During my PhD, I will study the mechanisms by which these VEGF-inhibitors might change the diameter of the isolated vessel. If these drugs cause a decrease in the vessel diameter, this could be an explanation for the increased blood pressure in patients. In this way, our knowledge on the mechanism behind the blood pressure raise will develop further, giving us more information on how to avoid this in the future.
References:
- Schjørring, O. L., Carlsson, R. & Simonsen, U. Pressure Myography to Study the Function and Structure of Isolated Small Arteries. Methods in Molecular Biology, vol. 1339, 277–285 (2015).
- Ferrara, N. & Adamis, A. P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 15, 385–403 (2016).
05/05/2021 - Martina (ESR #2)
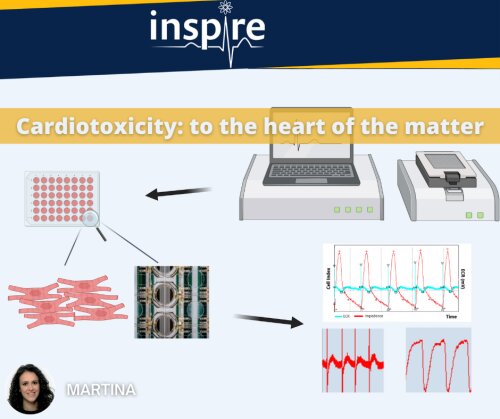
On average, every year around 37 new drugs are launched on the market with a cost of 1.5 billion dollars each. However, attrition rates remain high, allowing only 2% of potential candidates to enter clinical trials¹. The discipline of Safety Pharmacology aims to detect safety liabilities and adverse events early-on. While safety evaluation requires animal experimentation for certain steps, significant progress has been made to perform initial screenings on cellular assays. The Comprehensive In Vitro Proarrhythmia Assay (CiPA) initiative, established in 2013 by pharmaceutical industry and regulatory bodies is showing the value of cellular (“in vitro”) assays and computer models (“in silico”) to evaluate cardiotoxicity of drugs in the early stages of development, avoiding economical and time wastes ²,³. Moreover, based on the results of the CiPA initiative, a dialogue has been started to change the regulatory guidelines for non-clinical and clinical cardiac safety evaluation of new drugs.
This picture shows the system we are using at UCB Pharma to evaluate drug-induced cardiotoxicity on in vitro human induced pluripotent stem cell - derived cardiomyocyte (hiPSC-CM) cultures. The xCELLigence Real-Time Cell Analysis (RTCA) CardioECR System is a platform that provides powerful means to record cells in real time and in a non-invasive way. It allows to combine field potential recording and impedance for the measurement of integrated electrophysiology and contractility of cardiomyocytes, enabling the detection of changes in morphology, cell adherence and viability. Thanks to this system, we are able to quantify and predict drug cardiotoxicity in a 48-well format in the early phase of its development. The aim of my PhD project is to investigate the sensitivity of different cell models when treated with cardiotoxicants and to explore additional molecular biomarkers to further improve the predictive value.
Reference:
- IFPMA. IFPMA-Facts-And-Figures-2017. Int Fed Pharmacutical Manuf Asoc. 2017;
- Blinova K, Stohlman J, Vicente J, Chan D, Johannesen L, Hortigon-Vinagre MP, et al. Comprehensive translational assessment of human- induced pluripotent stem cell derived cardiomyocytes for evaluating drug-induced arrhythmias. Toxicol Sci. 2017;
- Blinova K, Dang Q, Millard D, Smith G, Pierson J, Guo L, et al. International Multisite Study of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Drug Proarrhythmic Potential Assessment. Cell Rep. 2018
28/04/2021 - Elham (ESR #7)

Nowadays, most people use wireless systems in their homes for connecting to the internet and other data transportation. However, this technique can be used for cardiovascular data acquisition and other physiological data evaluation.
Wireless monitoring with implantable telemetry microchip allows us to remotely collect animals' physiological data, such as cardiovascular parameters, anytime during undisturbed movement and social interaction during group housing. This technique increases animal welfare by allowing a continuous recording of their physiological and ECG parameters. The telemetry system consists of two major components:
- An implantable unit
- A receiver/antenna linked to a computer
During my Ph.D. project, I will investigate real-time telemetry data to qualify, cluster, and validate cardiovascular data in combination with video tracking to understand animals' social traits to reduce data variance.
Reference:
- Home. (n.d.). Retrieved February 03, 2021, from https://www.tse-systems.com/
21/04/2021 - Charles (ESR #9)

This image presents a Mass Spectrometry Imaging (MSI) section of a mouse heart. MSI enables to visualise biomolecules or chemical compounds on tissue sections. During the last decade, MSI has emerged as a powerful technique in drug development.
MSI can be used to investigate spatial distribution of a drug and its metabolites. In this context, we performed an image acquisition of hearts dosed with doxorubicin. Doxorubicin is an efficient anti-cancer drug that may cause dose-dependent cardiotoxicity. The image (top slide) shows three hearts of which two were collected 3 (middle) and 24 hours (right) post-doxorubicin injection. A colour scale of intensity (from blue-minimum to white-maximum) represents the concentration of doxorubicin. In a next step, this workflow can be complemented by MSI-guided proteomics and metabolomics approaches to estimate the impact of the drug within the tissue.
During my PhD-project, I will employ MSI and spatial -omics strategies to study drug distribution and the associated tissue response in the field of cardiovascular diseases, safety pharmacology and toxicology.
14/04/2021 - Christian (ESR #4)

This Stellar telemetry device developed by TSE Systems allows researcher to collect cardiovascular data in laboratory animals. It can be implanted in small as well as in larger animals. Its unique internal data storage capability gives the researcher the freedom to move the animals away from their usual home cage environment while maintaining data acquisition. This ensures simultaneous data collection of vital signs while performing phenotypical, physiological, pharmacological, behavioural, metabolic and inhalation studies.
During my PhD project, I will help to further improve the current system by adding a micro-GPS function to additionally measure behaviour and locomotor activity. This technology will advance research in socially housed animals, in which human interaction will be kept to a minimum. This in turn improves the relevance and quality of the collected data and - very importantly - the well-being of the animals. 1,2
This small telemetry device will have a huge impact on the flexibility to perform a broad range of social animal experiments, especially in the context of developing new and safer drugs.
References:
- Jennings, M., Prescott, M. J., & Joint Working Group on Refinement (Primates). (2009). Refinements in husbandry, care and common procedures for non-human primates: Ninth report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Laboratory Animals, 43(1_suppl), 1-4 https://doi.org/10.1258/la.2008.0071437.
- Ellegaard, L., Cunningham, A., Edwards, S., Grand, N., Nevalainen, T., Prescott, M., & Schuurman, T. (2010). Welfare of the minipig with special reference to use in regulatory toxicology studies. Journal of pharmacological and toxicological methods, 62(3), 167-183. https://doi.org/10.1016/j.vascn.2010.05.006
07/04/2021 - Patrizia (ESR #12)

This picture shows a rat heart isolated and perfused according to the Langendorff method. This technique permits to study the mechanical activity of isolated mammalian heart, allowing us to measure cardiac contractility (left ventricle pressure or LVP) by means of a latex balloon inserted into the left ventricle and connected to a pressure transducer, as well as coronary perfusion pressure (CPP), recorded by a pressure transducer placed in the inflow line 1. The electrocardiogram (ECG) is also recorded, providing information about the electrical activity of heart.
Unanticipated cardiovascular toxicities have shed light on the necessity to identify cardiovascular safety of drugs in a preclinical setting. In this context, the isolated Langendorff heart model is a useful method to evaluate the effect of drugs on haemodynamic, as well as on electrophysiological parameters. The significant sensitivity and specificity of such approach make it a method with high translational value 2.
During my PhD, I will use the Langendorff technique to assess cardiovascular safety liabilities of novel anti-cancer therapies, specifically receptor tyrosine kinase inhibitors (RTKI) targeting vascular endothelial growth factor (VEGF) signalling pathway 3. This model will complement my in vivo experiments in order to clearly identify the cardiovascular complications due to these drugs, as well as to investigate the mechanisms underlying such toxicities.
References:
- Bell, R.M., Mocanu, M.M. & Yellon, D.M. Retrograde heart perfusion: The Langendorff technique of isolated heart perfusion. Journal of Molecular and Cellular Cardiology 50, 940-950 (2011).
- Authier, S., Pugsley, M.K. & Curtis, M.J. Haemodynamic Assessment in Safety Pharmacology. Handb Exp Pharmacol 229, 221-241 (2015).
- Poster SPS meeting 2019, Barcelona
31/03/2021 - Callan (ESR #10)

Safety Pharmacology and Arterial Stiffness
Safety pharmacology is an essential part of drug development aiming to identify, evaluate and investigate undesirable properties of a drug primarily prior to clinical trials. Successful screening strategies to detect cardiovascular liabilities have been implemented, but there is room for further refinement 1. In this perspective, arterial stiffness is an interesting, blood pressure-independent factor to predict the risk of cardiovascular disease, but has not been widely considered in safety pharmacology. Historically, arterial stiffness was considered to reflect (passive) biomechanical properties of the artery wall, such as the ratio of compliant elastin versus stiffer collagen fibers. Our PHYSPHAR research group has developed a unique and proprietary organ set-up, as seen in the image (i.e., the Rodent Oscillatory Tension Set-up to study Arterial Compliance, ROTSAC) that enables ex vivo determination of intrinsic arterial stiffness, independent of confounding factors, such as blood pressure or heart rate 2. This novel technological platform holds potential to directly evaluate acute effects of chosen drugs, untangling their underlying mechanisms.
Could this be the tool us scientists have been waiting for? Join us on our journey to find out!
References:
- Guns, P. D., Guth, B. D., Braam, S., Kosmidis, G., Matsa, E., Delaunois, A.,Valentin, J. P. (2020). INSPIRE: A European training network to foster research and training in cardiovascular safety pharmacology. J Pharmacol Toxicol Methods, 106889. doi:10.1016/j.vascn.2020.106889
- Le loup, A. J. A., Van Hove, C. E., De Moudt, S., De Meyer, G. R. Y., De Keulenaer,G. W., & Fransen, P. (2019). Vascular smooth muscle cell contraction and relaxation in the isolated aorta: a critical regulator of large artery compliance .Physiol Rep, 7(4), e13934. doi:10.14814/phy2.13934
25/03/2021 - Elham (ESR #7)

The calculation of adequate sample size is crucial in any safety pharmacology study. Power calculation it is the process by which we calculate the optimum number of animals required to arrive at ethically and scientifically valid results. Power calculations uses mathematics/statistical equations, which describe the relationship between the expected effect size of biological interest, standard deviation (SD) of the data, significance level (usually p <0.05 ), desired power, sample size, and the alternative H0 (null hypothesis). A study with too small sample size may produce inconclusive and unreliable results.
As part of my PhD project, I will develop data analysis workflows that enable efficient exploration of values of control groups reported in historical safety studies. Moreover, we believe that a better understanding of factors inducing variation in historical studies may help to improve future study designs.
References:
- Fitzpatrick, R. (n.d.). Why is sample Size important? Retrieved February 03, 2021, from https://blog.statsols.com/why-is-sample-size-important/
- Kadam, P., & Bhalerao, S. (2010, January). Sample size calculation. Retrieved February 03, 2021, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2876926/
17/03/2021 - Haibo (ESR #3)
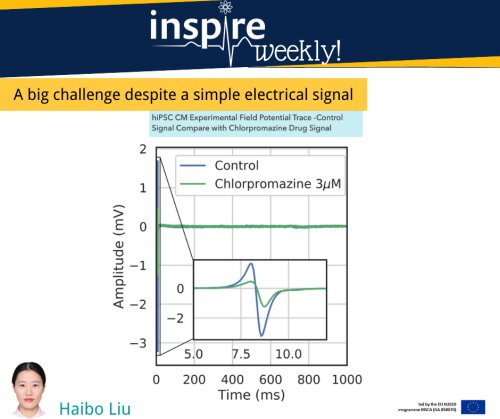
The picture shows an electrical signal
recorded from human heart muscle cells (cardiomyocytes). Interestingly, these
cardiomyocytes have been derived from human inducible Pluripotent Stem Cells
(hiPSC), a specific type of human stem cell obtained after reprogramming of
human skin cells (this topic will later be explained by ESR1 in another INSPIRE
Weekly contribution). hiPSC-based assays are used in drug development studies,
particularly for cardiovascular safety evaluation. Drug toxicity to the heart
could induce specific changes in the amplitude or shape of the signal, thereby
reflecting underlying mechanisms. However, manually analysing these signal data
is challenging given the very large amount of data generated by these
experiments.
My PhD research will focus on automatically analysing the large datasets from hiPSC-cardiomyocytes assays and developing computer-based prediction models (using artificial intelligence and machine learning) to detect and classify drug effects.
Reference:
- Raphel F, De Korte T, Lombardi D, Braam S, Gerbeau JF (2020). A greedy classifier optimization strategy to assess ion channel blocking activity and pro-arrhythmia in hiPSC-cardiomyocytes. PLOS Computational Biology 16(9): e1008203. https://doi.org/10.1371/journal.pcbi.1008203
03/04/2021 - Sara (ESR #8)
Blood pressure is a physiological parameter that can easily be measured and that correlates to one’s cardiovascular risk. Blood pressure is also a key factor to evaluate the cardiovascular safety of new drug candidates. Typically, blood pressure has a pulsatile pattern resulting in systolic (high) and diastolic (low) pressure values. An additional haemodynamic parameter includes pulse wave velocity (PWV), which is estimated based on pressure measurements at different locations in the body (e.g. arm versus foot). The PWV is a proxi of the stiffness (or reduced elasticity) of one’s larger arteries and is a good predictor of cardiovascular risk, independently of blood pressure. Further, using biophysics it is possible to derive from PWV the intrinsic arterial stiffness that may reflect pathological or drug-related changes of the vascular wall.
As part of my PhD, I will improve parameter estimation of biophysical quantities that cannot be measured directly in patients or lab animals. In fact, by combining pressure measurements and mathematical modelling as well as numerical simulation, we can estimate quantities like the Young modulus of the artery wall (or modulus of elasticity in tension). The Young modulus is a mechanical property that measures the tensile stiffness of a solid material and that will inform whether a disease (e.g. vascular calcification that occurs in dialysis patients) or a drug (cf. safety pharmacology) affects the biomechanical properties of our vascular system. Moreover, this detailed information is important to carefully assess and investigate the haemodynamic impact of changes in intrinsic stiffness.
References:
- Gerbeau, J., Lombardi, D., & Tixier, E. (2018). How to choose biomarkers in view of parameter estimation. Mathematical Biosciences, 303, 62-74. https://doi.org/10.1016/j.mbs.2018.06.003
24/02/2021 - Patrizia (ESR #12)
This picture shows some cardiovascular variables obtained through a method to determine blood flow called pulsed Doppler flowmetry and recorded using a computer-based system (IdeeQ). This technique allows us to make measurements of blood flow in three different vascular beds, while at the same time measuring heart rate and blood pressure, in conscious, freely moving rats. In order to evaluate these changes in the dynamics of the blood flow (haemodynamic), Doppler flow probes need to be surgically placed on the studied vessels. Several parameters will then be recorded using a computer-based system 1,2. Because the experiments are performed in a live rat, this method also permits us to measure haemodynamic changes in different parts of the vascular system, with reflex systems intact; these are often affected by anesthetics.
During my PhD project I will use the pulsed Doppler flowmetry to investigate changes in mesenteric, renal and hindquarters vascular conductance, as well as heart rate and mean arterial pressure, in rats treated with anticancer drugs targeting vascular endothelial growth factor (VEGF) signalling. Moreover, this approach will also provide essential information to clearly define the mechanism of action underlying cardiovascular impairment due to such novel targeted therapies used in cancer treatment. This project will give us more detailed information about the safety pharmacology issues associated with these treatments 3.
References:
- Haywood, J.R., Shaffer, R.A., Fastenow, C., Fink, G.D. & Brody, M.J. Regional blood flow measurement with pulsed Doppler flowmeter in conscious rat. American Journal of Physiology-Heart and Circulatory Physiology 241, H273-H278 (1981).
- Gardiner, S.M., Compton, A.M., Bennett, T. & Hartley, C.J. Can pulsed Doppler technique measure changes in aortic blood flow in conscious rats? American Journal of Physiology-Heart and Circulatory Physiology 259, H448-H456 (1990).
- Poster SPS meeting 2019, Barcelona
17/02/2021 - Marieke (ESR #11)
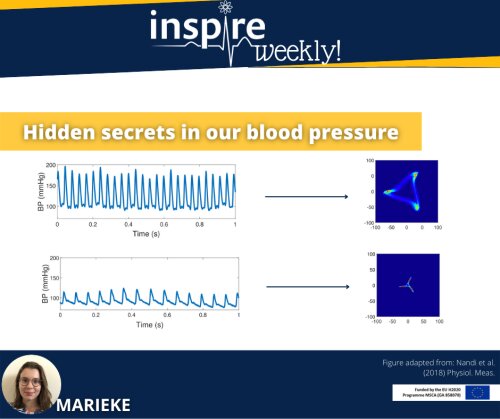
The measurement of blood pressure gives us valuable information on the health of a patient’s heart and blood vessels. The blood pressure is recorded as a waveform, a regularly repeating signal over time. Typically, the maximum and minimum value of the waveform are evaluated. In a healthy individual, these values are approximately 120/80 mmHg. In many diseases, the blood pressure is raised.
However, besides this maximum and minimum value, there is considerably more information captured in the recording of a blood pressure, that is often not taken into account. Analysis of the shape of the waveform and variability of this signal may give us additional information on how the heart and blood vessels are affected, particularly after drug treatment. In order to look at subtle changes in these waveform features, a new model was developed, called the attractor reconstruction. This mathematical model converts the blood pressure waveform (on the left-hand side of the flyer) into a 2D image (as shown on the right-hand side). By analysing different features of this 2D image, such as colour or length of the loops, we can extract detailed information on the effects of drugs on the heart and blood vessels. During my project, I will apply this attractor reconstruction on blood pressure waveforms and blood flow waveforms, to see how a number of anticancer drugs affect the cardiovascular system. In this way, I will explore the mechanism and safety of these medicines.
References:
- Nandi, M., & Aston, P. J. (2020). Extracting new information from old waveforms: Symmetric projection attractor reconstruction: Where maths meets medicine. Experimental Physiology, 105(9), 1444–1451. https://doi.org/10.1113/EP087873
- Nandi, M., Venton, J., & Aston, P. J. (2018). A novel method to quantify arterial pulse waveform morphology: Attractor reconstruction for physiologists and clinicians. Physiological Measurement, 39(10). https://doi.org/10.1088/1361-6579/aae46a
10/02/2021 - Charles (ESR #9)
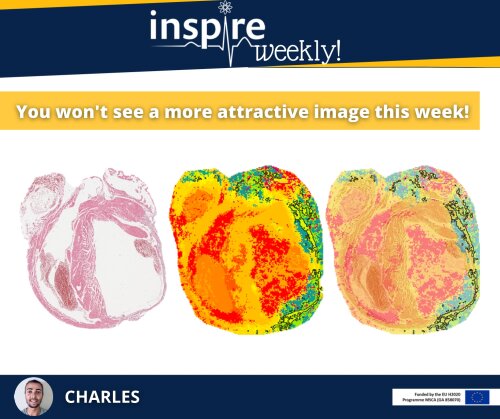
This image presents a usual workflow for data analysis by MALDI-MSI. MSI, standing for mass spectrometry imaging, is a label free technique allowing visualization of biomolecules on tissue sections. The image on the flyer shows heart sections obtained from an ischemia mouse model to study peptide distribution differences between ischemic (highlighted in black) and healthy regions. An overlay (right) of a conventional histology image (left) and a MSI image provides a better understanding of the molecular distribution within the tissue.
Segmentation analysis (middle) clustered the peptide spectra into different groups represented by different colors based on their molecular similarity, revealing a specific peptide signature in the ischemic area. This workflow is usually followed by MALDI-MSI guided proteomics for in depth protein identification.
During my PhD-project, I will employ MSI and spatial -omics strategies to study protein and glycan regulation in the field of cardiovascular diseases and toxicology.
03/02/2021 - Benji (ESR #13)
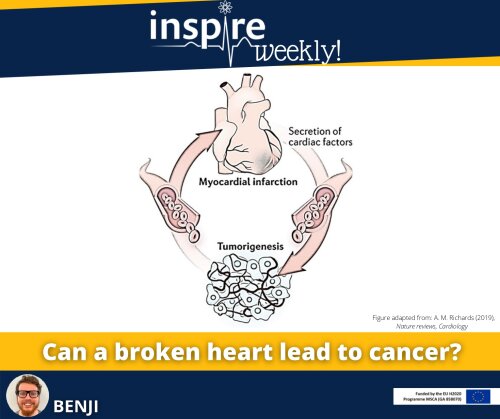
Historically, cancer and cardiovascular diseases were seen as separate entities only sharing several common risk factors, e.g. smoking, obesity and genetic background.1 Recent evidence, however, demonstrates that heart failure as a result of a heart attack promotes cancer development in mice.2 In the field of cardio-oncology, the toxic effects of anti-cancer drugs on the heart, eventually leading to heart failure or other cardiovascular adverse events, are being investigated extensively. However, the possibility of heart failure causing or worsening cancer growth is a novel discovery and is leading to the rise of the ‘reversed cardio-oncology’ field.
How does heart failure promote cancer growth? Several studies point towards proteins that are secreted by the damaged heart.2,3 These proteins are secreted by several biological processes with the aim to heal injuries, similar to wound healing. The ‘wound healing’ processes that are favorable in the heart, however, might promote cancer growth. During my PhD I will study the link between heart failure and cancer with a specific focus on endothelium-derived growth factors.
References:
- Avraham, S., Abu-Sharki, S., Shofti, R., Haas, T., Korin, B., Kalfon, R., Friedman, T., Shiran, A., Saliba, W., Shaked, Y., & Aronheim, A. (2020). Early Cardiac Remodeling Promotes Tumor Growth and Metastasis. Circulation, 142(7), 670–683. https://doi.org/10.1161/CIRCULATIONAHA.120.046471
- Meijers, W. C., Maglione, M., Bakker, S. J. L., Oberhuber, R., Kieneker, L. M., De Jong, S., Haubner, B. J., Nagengast, W. B., Lyon, A. R., Van Der Vegt, B., Van Veldhuisen, D. J., Westenbrink, B. D., Van Der Meer, P., Silljé, H. H. W., & De Boer, R. A. (2018). Heart failure stimulates tumor growth by circulating factors. Circulation, 138(7), 678–691. https://doi.org/10.1161/CIRCULATIONAHA.117.030816
- Moslehi, J., Zhang, Q., & Moore, K. J. (2020). Crosstalk between the heart and cancer: Beyond drug toxicity. In Circulation (Vol. 142, Issue 7, pp. 684–687). Lippincott Williams and Wilkins. https://doi.org/10.1161/CIRCULATIONAHA.120.048655
27/01/2021 - Dustin (ESR #14)

This image shows a size comparison of a regular pen and a pressure volume catheter used for precise analysis of the heart function in mice. This useful but tiny device can be inserted via a blood vessel inside the neck into the left ventricle (left chamber) to determine simultaneously the pressure and volume in the beating heart of a mouse (in vivo experiment). The catheter is connected to a computer allowing real time assessment of each heart beat generating pressure volume loops (PV-loops). PV-loops can be used to assess various parameters that characterize heart function.
Due to the small size of the mice and especially the mice heart, precise assessment of the cardiac function remains challenging. Nowadays, several imaging technologies are used to provide information regarding cardiac function in mice (e.g. ultrasonic or magnetic resonance imaging). However, only PV-loops provide the precise, real time and simultaneous measurement of chamber pressure and volume in mice heart. Also a great advantage of PV-loops is that they have already been well established in humans, thereby enhancing translation of the results to humans.1 During my PhD project I will use PV-loops to study the toxic effects of anti-cancer drugs in mice hearts.2 This gives a better insight into the side effects of anti-cancer drug and helps to develop new strategies to reduce them.
References:
- Townsend, D. W. (2016). Measuring pressure volume loops in the mouse. Journal of Visualized Experiments, 2016(111), 53810. https://doi.org/10.3791/53810
- Poster SPS meeting 2019, Barcelona