The overall aim of our group is to understand how neuronal circuits form early in development and the processes that can lead to malfunction or degeneration of these circuits later in life. We explore the interplay between genetic instructions and patterns of neural activity that guide the development and integration of neurons within neuronal circuits with the aim to understand how genetic mutations and environmental conditions can lead to neurological disorders. We use a combination of in vitro multi-neuron patch-clamp electrophysiology, optogenetic and pharmacogenetic techniques, in vivo electrophysiological recordings as well as behavioural and computational approaches to find answers. Together with the other groups within ENU we aim to understand the nervous system across multiple levels of organisation ranging from ion channels and individual neurons to complex neuronal circuits and behaviour.
Precision in connectivity within neural circuits is critical for their function. We recently focussed efforts in furthering our understanding how the connectivity between neurons in the striatum arise during early postnatal development. This led to observations that the excitatory inputs from cortex and thalamus onto the striatal spiny projection neurons (SPNs) as well as their lateral inhibitory connections are in place at the start of the second postnatal week, including clear biases in connectivity (e.g. preferred connectivity between D2 SPNs, Krajeski et al., 2019). We subsequently found that embryonic neuronal progenitor origin of SPNs contributes to aspects of the biased synaptic connectivity in the striatum, with SPNs in the dorsomedial striatum derived from apical intermediate progenitors preferentially receiving input from the medial prefrontal cortex and those derived from other progenitors preferentially receiving input from the visual cortex (van Heusden et al., 2021) and SPNs with a similar birth-date preferentially forming lateral inhibitory connections with each other. We recently started exploring how early patterns of neuronal activity in the 1st and 2nd postnatal week affect the development of these circuits and describe how the cortex appears to be the main driver of activity in the striatum (Klavinskis-Whiting). These early patterns of neuronal activity take the shape of intermittent bursts of high frequency activity fitting to initiate forms of synaptic plasticity.
Members
- Tommas Ellender, obtained his DPhil from the University of Oxford and subsequently he worked as a postdoctoral fellow in both the MRC Anatomical Neuropharmacology Unit and Department of Pharmacology (Oxford, UK) before starting his own lab.
- Phd. Danijela Bataveljic (postdoctoral fellow)
- Anezka Macey-Dare, PhD student (host institution: University of Oxford)
- Jack Gordon, PhD student (host institution: University of Oxford)
- Yasmin Crass, PhD student
- Yana van de Poll, PhD student
- Noortje Zonnekein, PhD student
Selected recent publications
From Progenitors to Progeny: Shaping Striatal Circuit Development and Function
From Progenitors to Progeny: Shaping Striatal Circuit Development and Function
Rhys Knowles, Nathalie Dehorter and Tommas Ellender
Journal of Neuroscience 17 November 2021, 41 (46) 9483-9502 Full text (Publisher's DOI) https://doi.org/10.1523/JNEUROSCI.0620-21.2021
Histamine, Neuroinflammation and Neurodevelopment: A Review
Carthy E. and Ellender T., (2021)
Front. Neurosci., 14 July 2021 | https://doi.org/10.3389/fnins.2021.680214
Diversity in striatal synaptic circuits arises from distinct embryonic progenitor pools in the ventral telencephalon.
van Heusden F. et al, (2021),
Cell Rep, 35 Full text (DOI uitgever): https://doi.org/10.1016/J.CELREP.2021.109041
Combining Whole-Cell Patch-Clamp Recordings with Single-Cell RNA Sequencing
ELLENDER T. and MAHFOOZ K., (2020),
Patch Clamp Electrophysiology Methods and Protocols, 2188, 179 - 189 Full text (DOI uitgever): https://doi.org/10.1007/978-1-0716-0818-0_9
Histaminergic control of corticostriatal synaptic plasticity during early postnatal development
Han S. et al, (2020),
J Neurosci, 40, 6557 - 6571 Full text (Publisher's DOI) https://doi.org/10.1523/JNEUROSCI.0740-20.2020
Embryonic progenitor pools generate diversity in fine-scale excitatory cortical subnetworks
Embryonic progenitor pools generate diversity in fine-scale excitatory cortical subnetworks
Ellender TJ. et al, (2019),
Nat Commun, 10 Full text (Publisher's DOI) https://doi.org/10.1038/S41467-019-13206-1
-
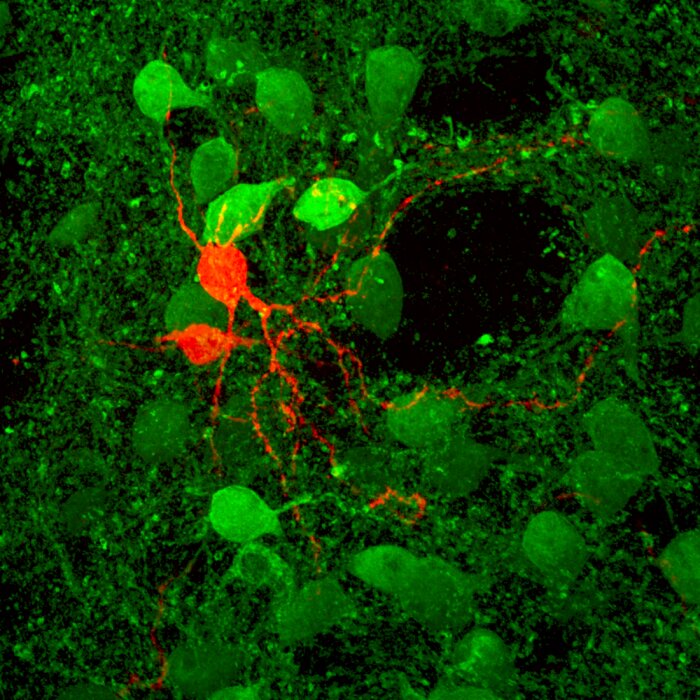 Striatal SPN paired recordings (Ellender et al. 2011)
Striatal SPN paired recordings (Ellender et al. 2011) -
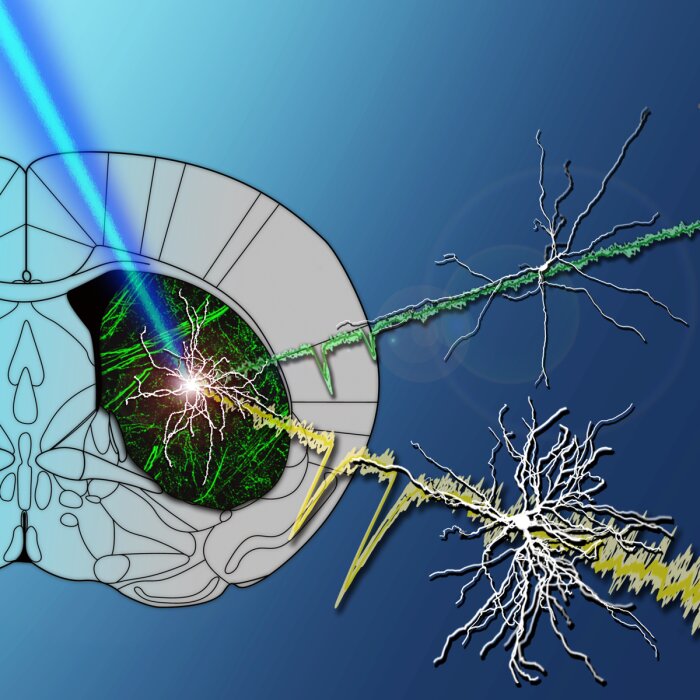 Optogenetic investigation of thalamostriatal afferents (Ellender et al. 2013)
Optogenetic investigation of thalamostriatal afferents (Ellender et al. 2013) -
 Striatal embryonic progenitors (van Heusden et al. 2021)
Striatal embryonic progenitors (van Heusden et al. 2021) -
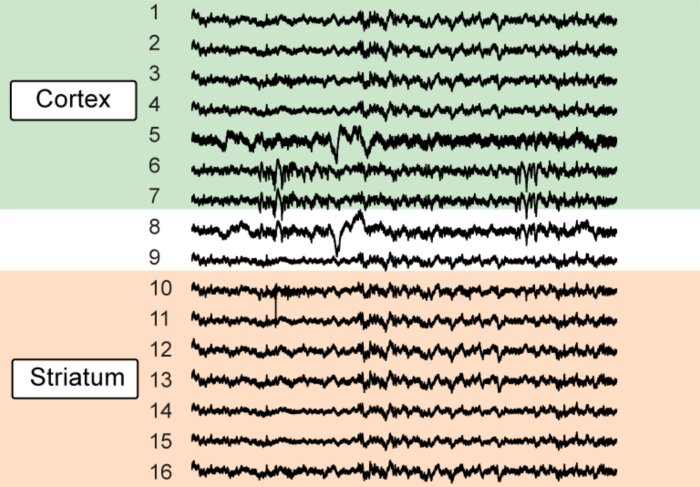 Early network activity (Klavinskis-Whiting et al. 2022)
Early network activity (Klavinskis-Whiting et al. 2022) -
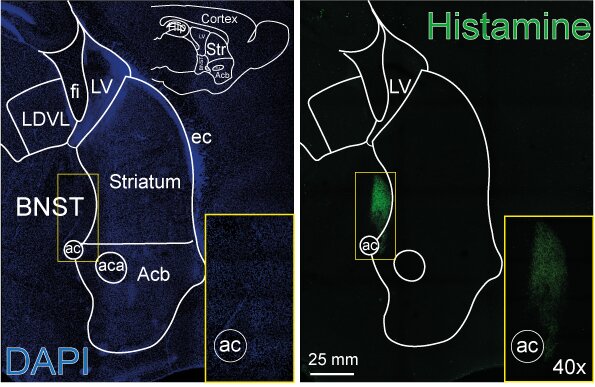 Histaminergic innervation of BNST (Marquez-Gomez et al. 2023)
Histaminergic innervation of BNST (Marquez-Gomez et al. 2023)