15/12/2021 - Callan (ESR#10)
In Safety Pharmacology, an important element of drug research and development, it is common practice to determine the blood concentration of a drug candidate over time. This is important information to understand the drug exposure-response relationship (i.e., PharmacoKienetic-Pharmacodynamic (PK-PD modelling)). Of note, only a free, unbound drug will interact with its molecular target. Furthermore, plasma concentrations can be used to investigate and calculate drug exposure of specific tissues. During drug development, PK-PD modelling will guide the researchers to determine the appropriate therapeutical dose, while avoiding adverse events due to exposure to toxic doses.
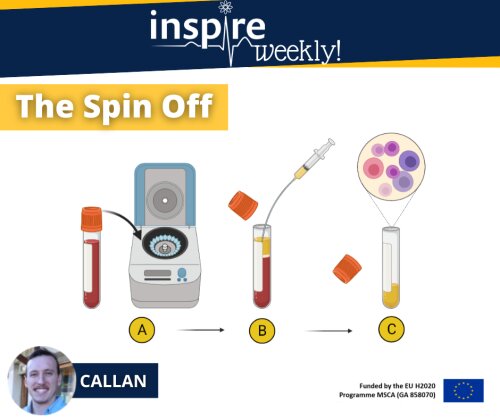
In this INSPIRE Weekly post, I explain the procedure to obtain plasma from collected blood samples:
The first step (A) involves placing the blood sample tube into a centrifuge. This device uses centrifugal forces to separate the various components within your blood whilst spinning them at high speed around its axis (Stephenson. 2010). The heavier and denser red blood cells will sink to the bottom of the tube whilst the lighter, less dense plasma will rise to the top of the tube. Leaving a clear separation between your red blood cells and your plasma as seen in step B.
The collection step (B) is done by making use of a pipette or syringe and drawing all the lighter less dense plasma (as depicted in yellow) from the tube.
The collected plasma (c) is then stored in a separate tube at -80°C until molecular analysis is performed to predict the concentration of the drug present in the plasma of the subject.
References:
- Rizk, M. L., et al. (2017). "Importance of Drug Pharmacokinetics at the Site of Action." Clin Transl Sci 10(3): 133-142.
- Frank H. Stephenson. (2010). “Calculations for Molecular Biology and Biotechnology.” (Second Edition).
- Tillement, J.-P. (1986). “Protein binding and drug transport: Symposium.” Schattauer.