05/05/2021 - Martina (ESR #2)
On average, every year around 37 new drugs are launched on the market with a cost of 1.5 billion dollars each. However, attrition rates remain high, allowing only 2% of potential candidates to enter clinical trials¹. The discipline of Safety Pharmacology aims to detect safety liabilities and adverse events early-on. While safety evaluation requires animal experimentation for certain steps, significant progress has been made to perform initial screenings on cellular assays. The Comprehensive In Vitro Proarrhythmia Assay (CiPA) initiative, established in 2013 by pharmaceutical industry and regulatory bodies is showing the value of cellular (“in vitro”) assays and computer models (“in silico”) to evaluate cardiotoxicity of drugs in the early stages of development, avoiding economical and time wastes ²,³. Moreover, based on the results of the CiPA initiative, a dialogue has been started to change the regulatory guidelines for non-clinical and clinical cardiac safety evaluation of new drugs.
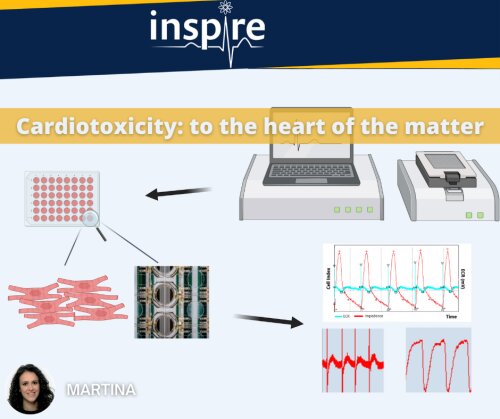
This picture shows the system we are using at UCB Pharma to evaluate drug-induced cardiotoxicity on in vitro human induced pluripotent stem cell - derived cardiomyocyte (hiPSC-CM) cultures. The xCELLigence Real-Time Cell Analysis (RTCA) CardioECR System is a platform that provides powerful means to record cells in real time and in a non-invasive way. It allows to combine field potential recording and impedance for the measurement of integrated electrophysiology and contractility of cardiomyocytes, enabling the detection of changes in morphology, cell adherence and viability. Thanks to this system, we are able to quantify and predict drug cardiotoxicity in a 48-well format in the early phase of its development. The aim of my PhD project is to investigate the sensitivity of different cell models when treated with cardiotoxicants and to explore additional molecular biomarkers to further improve the predictive value.
Reference:
- IFPMA. IFPMA-Facts-And-Figures-2017. Int Fed Pharmacutical Manuf Asoc. 2017;
- Blinova K, Stohlman J, Vicente J, Chan D, Johannesen L, Hortigon-Vinagre MP, et al. Comprehensive translational assessment of human- induced pluripotent stem cell derived cardiomyocytes for evaluating drug-induced arrhythmias. Toxicol Sci. 2017;
- Blinova K, Dang Q, Millard D, Smith G, Pierson J, Guo L, et al. International Multisite Study of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Drug Proarrhythmic Potential Assessment. Cell Rep. 2018