Malignant hyperthermia.
‘Malignanthyperthermia’ (MH) is a life-threatening, potentially fatal complication ofgeneral anesthesia triggered by the commonly used inhalational anesthesics andsuccinylcholine.
Introduction
In 1962, Denborough and Lovell described thecase of a 21 year old man in Melbourne, Australia, with a fractured leg who wasvery anxious about receiving general anesthesia because 10 of his familymembers had died during general anesthesia with ether. The anesthesiologists therefore decided tousing halothane, a recently introduced general anesthetic agent. After 10minutes of anesthesia, the patient became tachycardic, hypotensive, hypoxemic,and he developed a very high fever. He was packed in ice and halothane wasdiscontinued. The patient subsequently recovered uneventfully.
His family was investigated by Dr. M Denboroughwho noted that the pattern of deaths appeared to follow an autosomal dominantinheritance. Creatine phosphokinase (CK) levels were found to be elevated inmany of the family members. On the basis of these findings this original report alreadyhypothetized MH to be a metabolic myopathy with an autosomal dominantinheritance pattern.
Years of research have indeed shown MH to be a pharmacogeneticdisorder of skeletal muscle metabolism. Acute episodes of MH are precipitatedby exposure to inhaled fluorinated anesthetics e.g.halothane, enflurane, isoflurane, sevoflurane,desflurane, and/or succinylcholine resulting in a sustained increase inintramyoplasmic Ca+2. This induces afulminant activation of metabolism in striated muscle, and is expressed ashypercapnia, high temperature, generalized muscle rigidity, rhabdomyolysis,mixed acidosis, arrhythmias and possibly death. At a cellular level, MH is characterized byabnormalities in excitation-contraction coupling (ECC) and the control ofmyoplasmic calcium levels during muscle activation. This has been shown to be the result of defects in the genes coding for the proteinsinvolved in skeletal muscle excitation-contraction coupling, most often theryanodine receptor gene (RyR1) onchromosome 19q.
Physiology - Regulationof Calcium homeostasis.
Skeletalmuscle excitation is initiated by motor neuron stimulation at the neuromuscularjunction. This generates an action potential which travels along the musclemembrane and enters the interior of the muscle fibers by means of specialized transverse tubules (TT). The TT arefound at regular intervals at the long axis of the muscle fiber and theirpresence permits a coordinated skeletal musclecontraction.
The transverse tubules contain calcium channels thatare voltage activated, the so-called dihydropyridinereceptors (DHPR). This L-type voltage-dependentCa+2 channel (Ca1.1v) has multiple subunits (a1, a2, b, g, and d). Its a1 subunit - encoded by the geneCACNA1S on chrom 1- overlaps corresponding groupings of Ca-channels(RyR1-receptors) embedded in the wall of the sarcoplasmic reticulum (SR).
The 15.5 kb cDNA of the corresponding RyR1-gene on chromosome 19 encodes a5035 amino acid protein. Four identicalsubunits of 565 kDa form the homotetramic receptor in the SR-membrane. The 20%COOH-terminal end of the molecule forms transmembrane channel domains whereasthe NH2 terminal forms a large cytosolic protrusion which extends towards thetransverse tubule and makes contact with the DHPR.
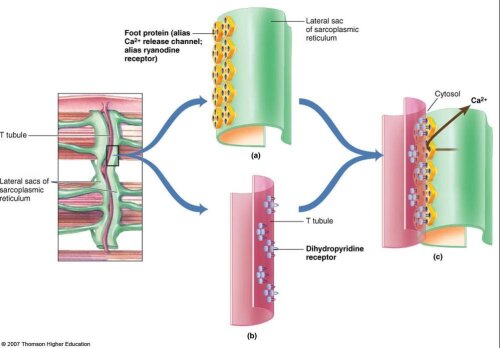
Depolarization of the DHPR receptors induces atri-dimensional conformational change which in turn is mechanically coupled tothe ryanodine receptor. The RyR1-channel opens and releases Ca+2. This Calcium-releasestimulates the contractile apparatus and shortening of the skeletal muscle.
Relaxationoccurs with the reuptake of Ca+2 into the SR by means of a Store-OperatedCalcium Entry (SOCE) and Excitation-Coupled Calcium Entry (ECCE) which contributeto the reuptake of Ca+2. Repolarization of the skeletal muscle membrane ismediated by fast inactivation of the Na+ channel, and opening of potassiumchannels generating an outwardly rectifying K+ current.
Physiopathology of MH
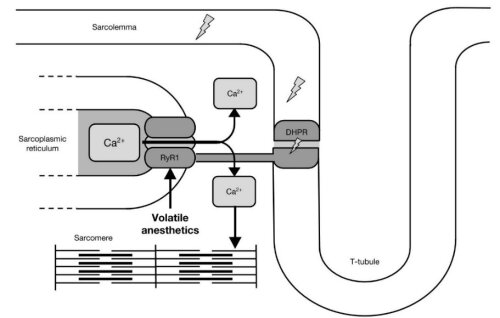
Ananesthetic-induced MH episode is manifested as a rapid and uncontrolled rise inmyoplasmic Ca2+, leading to sustained skeletal muscle rigidity andhypermetabolism and resulting in, hypercapnia, tachycardia, mixed acidosis andan abnormal rise in body temperature.
Ithas been demonstrated that a significant difference in calciumreleasing activity exists between MH-susceptible- and normal SR-preparations. Volatile anestheticscross the membrane and stimulate the RyR1-receptor resulting in a longer open time probability and thus an increased rate ofcalcium release triggering the hyperacute rhabdomyolysis.
Epidemiology
The incidence of an MH episode isestimated at 1 in 50,000 adults and 1 in 10,000 children and adolescentsundergoing general anesthesia. The incidence varies between reports and mainly depends on the frequencyof trigger anesthetics used, as well as the prevalence of susceptibilitymutations in the population.
Recent estimates suggest that theactual proportion of individuals carrying the MH trait (e.g. a mutated RyR1gene) is as high as 1 in 2000. The discrepancy between the rarity ofclinical crises and prevalence of RyR1 mutationsindicates the existence of incomplete penetrance, as well as the fact that anumber of MH crises are missed on the basis of their insidious clinicalcharacter. This explains why up to 50% of individuals with MH susceptibility haveundergone anesthesia uneventfully before despite use of one of the agents knownto trigger MH.
Clinical presentation:
Classicalfulminant MH presents with a combination of the following clinical andbiochemical signs: tachycardia, arrhythmias, increasing haemodynamicinstability, early tachy- and polypnea, pronounced hypercapnia, respiratory andmixed acidosis, generalized rigidity, hyperthermia, postoperative myalgia andmyoglobunuria, grossly increased CPK, and finally, systemic complications such as hyperkalemia,renal failure, disseminated intravascular coagulation and eventually cardiacarrest.
It isimportant to note that a considerable variability in the clinical presentation exists,and more and more late-onset, or insidious clinical presentations are reported.
Larach et al. devised a set of clinicaldiagnostic criteria for MH research: the Clinical Grading Scale (CGS). Differentialweighting is given to each of the manifestations of the syndrome. Howevervaluable this scale may be for research purposes, in the clinical setting thisscale is more difficult to interpret as too often not all clinical signs oradditional blood tests are performed, resulting in a false low score.
Malignant Hyperthermia(MH) Clinical Grading Score (CGS)
Scoring Rules
1. MH Indicators
Review the listof clinical indicators. If any indicatoris present, add the points applicable for each indicator while observing thedouble-counting rule below, which applies to multiple indicators representingthe same single process. If no indicator is present, the patient's MH score iszero.
2. Double-counting
If more than oneindicator represents a single process, count only the indicator with thehighest score. Application of thisrule prevents double-counting when one clinical process has more than one clinicalmanifestation. Exception: the score for any relevant indicators in thefinal category "other indicators" should be added to the totalscore without regard to double- counting.
Clinical Indicators for Use in Determining theMalignant Hyperthermia (MH) Raw Score
Process I: Rigidity
Indicator | Points |
Generalized muscularrigidity (in absence of shivering due to hypothermia, orduring or immediately following emergence from inhalationalgeneral anesthesia) | 15 |
Masseter spasm shortlyfollowing succinylcholine administration | 15 |
Process II: Breakdown
Indicator | Points |
Elevated creatine kinase >20,000 IU after anesthetic that included succinylcholine | 15 |
Elevated creatine kinase >10,000 IU after anesthetic without succinylcholine | 15 |
Cola colored urine in perioperative period10 | |
Myoglobin in urine >60 g/L | 5 |
Myoglobin in serum >170 g/L | 5 |
Blood/plasma/serum K+ > 6 mEq/L (in absence of renal failure) | 3 |
ProcessIII: Respiratory Acidosis
Indicator | Points |
PETCO2>55mmHg with appropriately controlled ventilation | 15 |
Arterial PaCO2>60mmHg with appropriately controlled ventilation | 15 |
PETCO2>60 mmHg with spontaneous ventilation | 15 |
Arterial PaCO2>65mmHg with spontaneous ventilation | 15 |
Inappropriatehypercarbia (in anesthesiologist's judgment) | 15 |
Inappropriatetachypnea | 10 |
Process IV: Temperature Increase
Indicator | Points |
Inappropriately rapidincrease in temperature (in anesthesiologist's judgment) | 15 |
Inappropriately increased temperature > 38.8°C (101.8F)in the perioperative period(in anesthesiologist's judgment) | 10 |
Process V: Cardiac Involvement
Indicator | Points |
Inappropriate sinustachycardia | 3 |
Ventriculartachycardia or ventricular fibrillation | 3 |
Other indicators thatare not part of a single process
Indicator | Points |
Arterial base excessmore negative than 8 mEq/L | 10 |
Arterial pH <7.25 | 10 |
Rapid reversal of MHsigns of metabolic and/or respiratory acidosis with IV dantrolene | 5 |
3. Interpreting the raw score: MH rank and qualitative likelihood
Raw Score Range | MH Rank | Description of Likelihood ofMH |
0 | 1 | Almost never |
3-9 | 2 | Unlikely |
10-19 | 3 | Somewhat less than likely |
20-34 | 4 | Somewhat greater than likely |
35-49 | 5 | Very likely |
50+ | 6 | Almost certain |
Moleculargenetics
Previous research using the pig model e.g. ‘theporcine stress syndrome’ demonstrated that MH in these particular pig breeds is inheritedas an autosomalrecessive disorder based on the presence of one single dominantmutation (HAL-1643) on chromosome 6. Porcine Stress Syndrome associated with thehalothane gene makes pigs more susceptible to stress and stress-relateddeath. This problem leads to severely reducedmeat quality (pale, soft and exudative or 'PSE' pork) which represents a significanteconomic loss from the disease. A DNA-PCR is available toaccurately detect heterozygous (carrier) pigs.
The presence of synthenic groups of genes oriented theresearch on human MH towards chrom 19q13. Epidemiological studies have shown that - in the majority of families at least - susceptibilityto MH is transmitted in an autosomal dominant way, and not recessive as in thepig model. During the earlier years thehope was to find a single mutation, the presence of which could be used tounequivocally determine the MH-status of an individual by means of a ‘simplebloodtest’.
In 1990 the primary gene locus for MH was identifiedas the RyR1 gene on chromosome19q13.1. However, in contrast to thepig model of MH, ensuing research in humans has demonstrated both locus andallelic heterogeneity which very much complicates the genetic diagnosis.
Locus heterogeneity (different genes): Two major gene mutations causing MHS have beenidentified:
- MHS1 locus; OMIM 145600. The MHS1 locus encodesthe type 1 ryanodine receptor of muscle. RyR1 mutationsare identified in up to two thirds of individuals with confirmed MHS.
- CACNA1S (MHS5 locus) encodes the α1-subunit of the skeletal muscle dihydropyridine receptor L-type calcium channel. Mutations in CACNA1S account for 1% of all MHS.
- Very rare variants in several additional genes (CACNB1, CASQ1, SERCA1, CASQ2, KCNA1, Stac3) encoding proteins involved in calcium homeostasis have been reported but their true association with MH needs further validation.
Allelic heterogeneity (same gene,different mutations).
Todate there are more than 400 RyR1variants associated with MH-susceptibility. Amajor cause of this complexity is that RyR1 is a huge genecomprising 106 exons which encode 5,038 amino acids with a cDNA ~ 15,117b. It is one of the largestgenes in the human genome. The majority of gene mutations reported aremissense changes e.g.single amino acids in important regions of the ryanodine which alter thestructure of the RyR1 channel, causing it to open more easily and close moreslowly in response to the triggering drugs, resulting in a prolonged ‘opentime’ of the receptors and increased calcium-release.
The majority of these mutations cluster in 3‘hot spots’: MH/CCD region1 between amino acids 35 and 614 ((corresponding to exons 2, 6, 9, 11, 12, 14, 15, and17), MH/CCDregion 2 between amino acids 2129 and 2458 (corresponding to exons 39, 40, and 44–46), and a third domain (MH/CCD region3) at the highly conserved C-terminal region, which encodes theluminal/transmembrane domain of the protein that forms the ion pore (amino acidresidues 4668 and 4906). These are predicted to reside atthe myoplasmic foot region of the protein. Only 48 of the 400 reported mutations have beendemonstrated to be causative in an experimental setting and are considered tobe ‘true or diagnostic mutations’. Thesearepathogenic as a result of replacement of generally conserved amino acids thatlead to altered protein function. Other mutations – depending on their location - have repetitivelybeen found in several families and may or may not affect amino acid sequence or functionin the final protein (called VUS, Variants of Unknown Significance). Finally, harmless benign variants (polymorphisms) are foundin more than 1% of the population. New computational tools are developed suchas SIFT and PolyPhen to assess if a particular DNA variant is prone to be toleratedas harmless, or not be tolerated. All in all these findings have complicated themolecular genetic diagnosis of MH in a major way.
RyR1 mutations havealso been associated with other myopathies such as central core disease (CCD), multiminicoredisease (MmD), nemaline rod myopathy, congenital fibre type disproportion andhypokalemic periodic paralysis. Interestingly,mutations in RyR1 can produce asingle disease state, such as MH susceptibility or CCD alone, or a combinationof disease, i.e., MH susceptibility and CCD together. Central Core Disease (CCD) is the mostprevalent congenital myopathy characterized by moderate muscle weakness,musculoskeletal abnormalities, and characteristic histopathology. This myopathyis usually considered dominant, although recessive inheritance and allelesilencing in muscle have been reported. Complexheterozygosity can even lead to the co-existence of these diseases in onesingle family.
In vitro diagnosis
A family’s disposition to MH is identified by a clinicalMH episode in the proband, e.g. the first individual affected in a family.Until now, the in vitro contracture test (IVCT) of biopsied skeletal musclerepresents the only generally accepted method to confirm the MH disposition.
The EMHG (European Malignant HyperthermiaGroup) diagnostic Testing: http//www.emhg.org :
Susceptibility to MHis diagnosed by in vitro contracture testing (IVCT) with caffeine andhalothane. The IVC-test requires a sample of skeletal muscle tissue which isexposed in vitro to incremental doses of different specific testing agents andthe contracture response measured. The test is consideredpositive (MHS) if a sustained contracture of at least 2 mN is obtained in two different musclebundles at caffeine concentrations of 2 mM or less, and halothane concentrationsof 2 Vol% or less. Normal individuals(MHN) do not react at the threshold concentrations of either agent. Part of the patients only show an abnormalcontracture response to one of the two test-drugs. This is felt to reflect the phenotypicalvariability.
This test has beenstandardised across Europe by the EMHG and shows a high degree of sensitivity(99%) and specificity (93.5%).
The MH-laboratory of the University of Antwerpis the national reference centre for MH-diagnosis and accredited member of theEuropean Malignant Hyperthermia Group (EMHG).
Treatment, prevention and patient safety
According to recent North American clinicalstudies MH has a morbidity rate of 35% and a mortality rate of about 12%. Patientswho are known to be MH susceptible may be anesthetized with regional anesthesiaor local anesthesia without problems. If general anesthesia or sedation isrequired, the potent volatile agents and succinylcholine have to be avoided,and replaced by total intravenous anesthesia withpropofol/morphinomimetic/muscle relaxant. Preoperatively, the anesthesia workstation hasto be flushed with a fresh gas flow of 10L/min for at least 60 min, and thevaporizer has to be taken out of the circuit. A recent alternative is to use activated charcoal filters which veryeffectively remove traces of volatile anesthetics from the breathing circuit(VaporClean filters).
The only drugapproved for the treatment of an episode of MH is dantrolene. This drug wasdescribed over 40 years ago and widely used in the management of spasticitybefore its efficacy in treating MH was discovered.
This direct-actingmuscle relaxant has been shown to bind to RyR1 at a site distinct from theryanodine binding site and to inhibit RyR1-dependent Ca+2 transients, therebyserving to decrease resting Ca+2 in the myoplasm, as well as decrease the Ca+2transient after electrical excitation or exposure to caffeine or halothane.However, the exact mechanism by which this compound exerts its pharmacologicaleffects on RyR1 channel properties is still debated.
Dantrolene acts as a postsynaptic muscle relaxant, anddoes not inhibit RyR2-receptors which explains why the drug has no negativeinotropic effects.
The compound is highly lipid soluble, and waterinsoluble, which led to the delay of the development of the IV formulation.Mannitol and sodiumbicarbonate help to solubilize dantrolene but the resultingalkaline solution is irritating to peripheral veins and should therefore beinjected into a large vein or a fast running infusion.
Dantrolene (Dantrium – Norgine) is given in anintial dose of 2 mg/kg and repeated if clinical signs such as tachycardia,fever and high ETCO2 persist for more than 15 minutes.
A recent – but more expensive – alternative isRyanodex, which is a nanosuspension of dantrolene, supplied in 20 ml vialscontaining 250 mg of dantrolene. Ryanodex requires significantlyless IV fluid to reconstitute and is administered in less than 1 minutecompared to the 10 – 15 min for Dantrium. Ryanodex is not commercialized in Europe.
Overdosing results in CNSdepression, hypotension, nausea, vomiting and muscle weakness which lasts forup to 72 hours. Dantrolene may interact with a number of other drugs e.g.calcium channel blockers, potentiates non-depolarizing neuromuscular blockingagents, and the sedative action of benzodiazepines or other CNS-depressants.
MH susceptibility and relationship to othermuscle disorders
Over the lastdecade there has been a considerable increase in the number of patients in whomMH- susceptibility is thought to be associated with a second disease. This is especially true for a number ofneuromuscular disorders (e.g. myotonia congenita, Duchenne dystrophy, centralcore disease, hypokalemic periodic paralysis) and disease entities such asneuroleptic malignant syndrome, exertional heat stroke and others.
For severalreasons, the significance of this ‘association’ has been the cause of much debate. The main problems for interpreting a possibleassociation are several: the clinical signs of an MH-episode are aspecific, theclinical documentation and appropriate investigations are often either absentor insufficient, and last but not least the complexity of the laboratoryconfirmation of MH susceptibility does not simplify this matter.
The propensityof some dystrophic patients to react adversely to anesthetic agents includinglife-threatening rhabdomyolyis is well known but is primarily based on ‘toxic’effects of the anesthetic drugs on the fragile sarcolemma, called‘anesthesia-induced-rhabdomyolysis’. Other neuromuscular disorders may also predispose to anesthesia-relatedcomplications in their own right but a definite – and complex - associationonly appears to exist for the congenital myopathies (Central core disease,Multiminicore disease, centruclear myopathy, nemaline myopathy, congenitalfiber type disproportion,…). Most – butnot all – of these patients appear to be MH susceptible by standard in vitrocontracture (IVC) testing.
In contrast toMH, neuroleptic malignant syndrome arises from an imbalance of central nervoussystem neurotransmittors. This syndromeis felt to be different from MH.
The exactnature of the possible association with heat stroke is unclear but a subgroupof these patients may have an underlying skeletal muscle abnormality that couldlead to abnormal responses in the IVC test.
At the presenttime, more or less convincing evidence exists for the association of MH-susceptibilitywith other “RYR1-myopathies” such asCentral- and Multiminicore disease, King Denborough syndrome, and a subgroup ofpatients with exertional rhabdomyolysis. In about 15% of these patients RyR1-mutations have been found. For other diseases theassociation is considered to be weak.
References:
Denborough MA, Forster JF, Lovell RR, Maplestone PA, VilliersJD. Anaesthetic deaths in a family. Br J Anaesth 1962; 34: 395-396.
Rosenberg H. et al. Review Open Access Malignant hyperthermia.OrphanetJournal of Rare Diseases 2007, 2:21
Klingleret al. Functional and genetic characterization of clinical malignanthyperthermia crises: a multi-centre study. Orphanet Journal of Rare disease2014, 9:8.
Riazi S. MalignantHyperthermia in Canada: Characteristics of Index Anesthetics in 129 MalignantHyperthermia Susceptible Probands. Anesth Analg 2014; 118:381–7
Krause T, GerbershagenMU, Fiege M, Weisshorn R, Wappler F. Dantrolene--a review of its pharmacology,therapeutic use and new developments. Anaesthesia. 2004; 59:364–73.
Rosenberg H. Malignant hyperthermia: a Review. Orphanet J Rare Dis. 2015 Aug 4;10:93.
Riazi S, Kraeva N, Hopkins PM.Riazi S, et al.Malignant Hyperthermia in the Post-Genomics Era: New Perspectives on an OldConcept. Anesthesiology. 2018 Jan;128(1):168-180