Members
ZAP
BAP
Dr. Geert Van Hemelen
Dr. Jan Van de Perre
Research
- Mesenchymal stem cell therapy in the treatment of alveolar cleft defects
PhD A. Khojasteh (jan 2013)
In samenwerking met Shahid Beheshti universiteit (Tehran, Iran)
1. Overview of the research (introduction, methods, results, discussion)
TISSUE ENGINEERING OF ALVEOLAR CLEFT DEFECTS
Introduction
The vast majority of defects in bone tissue could heal spontaneously with minimal therapy; but in some cases including large bone defects such as alveolar cleft, it requires further treatment. Secondary alveolar bone grafting is an essential part of the management of cleft palate patients. The commonest site for acquiring autogenous bone for grafting has been the anterior iliac crest. However, its disadvantages including its limitation, painful surgery, risk of infection, bleeding, nerve damage and loss of function have made the researchers to look for an alternative. To reduce donor site morbidity and enhance bone regeneration, growth factors such as bone morphogenic proteins (BMPs) and platelet derived growth factors (PDGF) in combination with different kinds of bone substitutes were examined [1].
With the advent of in vivo tissue engineering methods, mesenchymal stromal cells and osteoprogenitor cells were also considered as a possible treatment solution since they were capable of increasing the number of potentially osteogenic cells. Bone marrow aspirate soaked in absorbable collagen sponge was reported as an alternative method for the closure of human alveolar clefts [2]. Mesenchymal stem cells (MSCs) different kind of bone substitute showed enhancement of the bone regeneration in alveolar cleft defect but not reach to complete healing [3,4]. Here we are going to increase new bone formation in alveolar cleft defect by modification of the scaffolds, enhancing delivery methods and adding some autogenous part to mesenchymal stem cells in alveolar cleft defects patients
Harvesting, identification and differentiation of mesenchymal stem cells from dental pulp and Buccal Fat Pad
We used dental pulp and buccal fat pad as a donor site for harvesting and differentiation of mesenchymal stem cells.
Dental Pulp
Two vital third molars of a healthy adult subject (21 year-old male) were extracted. Before extraction, the patient was asked to rinse with 0.2% chlorhexidine mouthwash (Behsa® Co., Tehran, Iran) for 1 min. After extraction, the teeth were immersed in physiological solution containing antibiotics.
The crown of each tooth was fractured in several parts and the pulp was uncovered. Dental pulp tissue was removed with a barbed broach. Dissected tissue was minced and incubated in 3 mg/ml type I collagenase and 4 mg/ml dispase in PBS (GIBCO Laboratories, Grand Island, NY, USA) at 37°C for 30 min. Then, the tissue fragments were immersed in α-MEM culture medium supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics (GIBCO) and placed in 25- cm2 flasks. Flasks were incubated in humidified atmosphere containing 5% CO2 at 37°C. Culture medium was changed twice a week and after reaching the subconfluent stage, the cells were removed by enzymatic digestion (0.25% trypsin–EDTA) and passaged. DPSCs of the third to fourth passage were subjected to the experiments.
Buccal Fat Pad
Buccal fat pad was harvested from healthy donors through the vestibular incision distal to the maxillary second molar. The fat pad was exposed with the blunt dissection while preserving the thin covering membrane. 3-5 ml were excised and delivered to the lab in DMEM medium. The tissue processing and cultivation of the stem cells has been done with the same methods with dental pulp. Adherent cells were expanded as monolayer cultures in a 5% CO2/ 95% air atmosphere at 37°C with medium changes every 3 days. The confluent cells were dissociated with trypsin and subcultured in new 6-well culture dishes at a plating density of 6×104 cells/dish. The cells could be seen under light microscopy (Fig.1)

Fig.1 Stellate Mesenchymal cells under light microscopy
IN VITRO OSTEOGENIC DIFFERENTIATION
Differentiation Staining
Alizarin Red Staining
To evaluate in vitro osteogenic potential of the isolated cells, confluent third passage culture was used. For this purpose, the osteogenic medium consisted of the control medium supplemented with 0.2 mM ascorbic acid (Sigma, St. Louis, MO), 10 mM Na-b-glycerophosphate (Sigma), and 108 M dexamethasone (Sigma) was substituted as culture media. The cultures were then incubated at 37 0C and 5% CO2 for 21 days. At the end of the cultivation period, the cells were fixed with 10% formalin for 10 minutes and stained with alizarin red (Sigma) for 15 minutes at room temperature so that the mineralized matrix of the bone could be examined. After 3 weeks, differentiated cells were fixed for 1 h with 4% formalin and rinsed with PBS (Gibco). Mineralization of the extracellular matrix was visualized by staining with 40 mM Alizarin Red S, pH 4.2, for 5 min (Fig. 2).
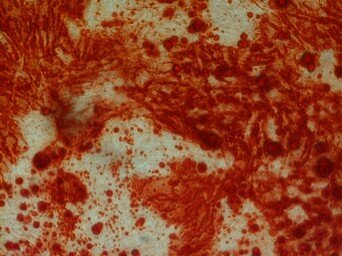
Fig.2. Alizarind Red Staining
Adipogenesis
To induce adipose differentiation, passaged-3 confluent culture was treated with the medium containing 100nM dexamethazone (Sigma) and 50 mg/ml indomethasine (Sigma). After 3 weeks, the cells were fixed with 4% formalin at the room temperature, washed with 70% ethanol and stained by oil red solution in 99% isopropanol for 15 minute (fig.3)
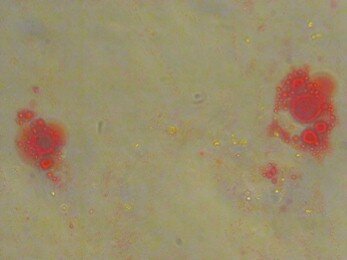
Fig.3. Oil Res Staining
RNA extraction and RT-PCR analysis of gene expression
The differentiation ability of the cells and some specific gene expression were also studied by RT-PCR analysis. The cells were also used for RNA extraction and reverse transcriptase–polymerase chain reaction (RT-PCR) analysis of osteocytic gene expression. According to the results, osteopontin, Col I A1, and mRNA were largely produced after the third week in an osteoinductive medium
2. Finding an appropriate scaffold for the cell delivery
Ceramics, Polymers, organics and composite material used to deliver cells in tissue engineering for the next year we are going to compare the effectiveness of each kind in proliferation and differentiation of mesenchymal stem cells by the following protocols
Cell Seeding:
Cells will stained with trypan blue (1:1; v/v) and count in a counting chamber using a light microscope (Nikon, Japan). A seeding concentration of 1.3x106 cells/mL will be cultured on various bone grafts. Each layer of scaffolds covering one of 24-well plates will be seeded with 2x105 MSCs in 200 μl of culture medium. The plates will be incubated for 1 h at 37°C allowing cell adhesion; then, half of the samples will receive 500 ml of standard medium (containing α-MEM supplemented with 10% FBS, 1% antibiotic–antimycotic solution and 2 mmol/l L-glutamine) and the other half will be cultured in 500 ml osteogenic medium (standard medium plus 10 ηm dexamethasone, 10 ηm β-glycerophosphate, and 50 μg/ml ascorbic acid).
SEM:
Scaffold surface topography, adherent cell morphology and extracellular matrix culture will be assessed using scanning electron microscopy (SEM). The seeded scaffolds will be rinsed in PBS solution twice, fixed in 2.5% glutaraldehyde for two hours, and then post-fixed in 1% osmium for another two hours. Then, samples will be dehydrated in graded concentrations of alcohol (30, 50, 70, 90, 95, and 100% ethanol). Afterwards, the samples will be air dried in a desiccator overnight, sputtered with gold and observed under a SEM (VEGA, TESCAN Czech Republic) at 10kV. Images of non-seeded scaffolds will also be taken. Moreover, the size of micro and macro porosities of each scaffold was measured by ImageJ 1.46 computer software (Wayne Rasband, NIH).
MTT assay:
After cultivation of the cell-seeded scaffolds for 24 hours, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma) test will be used. First, granules will be transferred to new wells in a 24-well plate in order to avoid counting adherent cells to the bottom of the plate. Then, serum-free-medium containing 10% MTT will b added to the wells and plates will be incubated at 37°C for 4 h. Next, dimethyl sulfoxide (CARLO ERBA, Italy) will be added to each well and incubated for one night. The supernatants will be collected and transferred to 96-well plate. Then, the absorbance at 590 nm will be determined in a microplate reader (Anthos, Austria) with a reference wavelength of 620 nm.
DNA assay:
The proliferation rate of the MSCs on different kind of scaffolds will be assessed by measuring the total DNA content of the cell-loaded scaffolds with AccuPrep Genomic DNA Extraction kit (BioNEER, Seoul, Korea) according to the manufacturer's protocols after 1, 7 and 14 days after cell seeding. After extraction of the DNA, the absorbance of each sample will be measured in duplicate at 260 nm with a spectrophotometer and DNA amounts will be calculated from a standard curve.
RT-PCR:
RT-PCR will be performed to evaluate the effect of the scaffolds on expression of osteogenic genes during stem cell differentiation. MSCs grow in standard and osteogenic medium will be used as negative and positive controls. In our previous experiments, we chose osteonectin (ON) as an early marker and osteocalcin (OC) and osteopontin (OP) as late markers of osteoblastic differentiation. ON is an important protein for osteoblast formation, maturation, and survival which remains at same level through osteoblast differentiation. OC and OP synthesis, however, rise during differentiation of osteoblasts and mineralized matrix production. OC is expressed in relation to matrix mineralization after proliferation and considered as an indicator of osteoblast maturation. In contrast to OC, OP is maximally expressed during proliferation phase and late osteoblast development.
Total RNA will be isolated using the RNeasyPlus Mini Kit (Qiagen, USA) and cDNA will be synthesized from 1 µg of the total RNA by cDNA synthesis kit (Bioneer, Korea) following the manufacturer’s instructions. ON, OC, and OP mRNA expression will be evaluated by RT-PCR using specific primers: ON (Forward: CCA AGT AAG TCC AAC GAA AG and Reverse: GGT GAT GTC CTC GTC TGT A; product length 349 bp), OC (Forward: CATGAGAGCCCTCACA and Reverse: AGA GCG ACACCCTAG AC; product length 292 bp), and OP (Forward: GGA AGA AAC TGT GGC AGA GGT GAC and Reverse: TGT TGT CCT CAT CCC TCT CAT ACA G; product length 464 bp). The β-actin (Act) will be used as housekeeping gene internal control (Forward: GTTGCTATCCAGGCTGTGC and Reverse: CATAGTCCGCCTAGAAGC; product length 739 bp). The PCR reactions will be performed using the following program: 95ºC for 2 min, 35 cycles of denaturation at 95ºC for 30 sec, annealing at 55° (OP and OC) and 61°C (ON and Act) for 30 sec, and extension at 72 ºC for 30 sec. PCR will be performed with a conventional PCR machine (Thermo Hybaid Sprint, UK). The PCR products will be electrophoresed on 1.5% agarose gels and viewed with UV illumination. The band density of each gene on agarose gel will quantified using Scion Image software (Scion Inc., Gaithersburg, MD, USA).
References:
1. Khojasteh A, Behnia H, Dashti SG, Stevens M. Current trends in mesenchymal stem cell application in bone augmentation: a review of the literature. Journal of Oral and Maxillofacial Surgery 70 (4), 972-982
2. Herford AS, Boyne PJ, Rawson R, Williams RP: Bone morphogenetic protein-induced repair of the premaxillary cleft. J Oral Maxillofac Surg 65: 2136e2141, 2007
3. Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Khoshzaban A, Atashi A: Secondary repair of alveolar clefts using human mesenchymal stem cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108(2): 1e6, 2009
4. Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Atashi A. Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: A preliminary report. Journal of Cranio-Maxillofacial Surgery 40 (1), 2-7
Ongoing clinical research
- Long term results on cleft, lip & nose reconstruction
- Long term results on soft & hard palate reconstruction following prof. Nadjmi’s technique of speech and mid facial growth
- Long term : skeletale stabiliteit na kaakcorrectie